the matrix, reloaded
by Neil Caudle
Dan and Agneta Simionescu found magic in the matrix, and it’s helping them fashion a new way to heal.
Today, Dan and Agneta Simionescu have branched out, running separate labs.
But they still use the word “we” when they talk about their work. How do they manage it—working so closely together, after all of these years? Aggie smiles, considers the question for a moment, and laughs. “I don’t really know how we do it,” she says, “but we do.”
“We always talk,” she adds, “and the work is exciting for us both. When we go to conferences and take notes, very often we write down the same things, because the same things are interesting for us. I think we complete each other.”
—Dr. Chris Wright
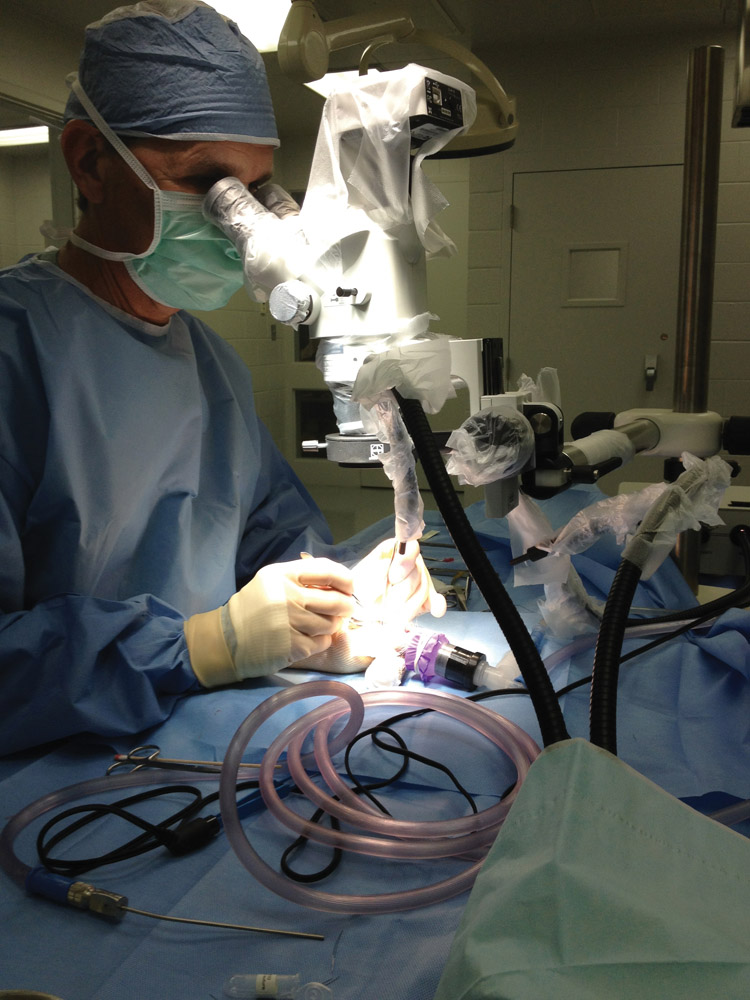
Dr. Fred Nelson performs brain surgery on a rat at Clemson’s Godley-Snell Research Center. With his help, the researchers are testing implants for replacing stroke-damaged brain tissue. Photo courtesy the Simionescu lab.
Surgeons help guide the research
Lots of research programs show promise, but not many are so promising that ten busy surgeons volunteer their time to contribute. That’s the case with the tissue-regeneration studies led by Dan Simionescu.
“What Dan and his team are doing is incredible,” says Dr. Chris Wright, a thoracic surgeon and chief of medical staff affairs for the Greenville Health System (GHS). “More than anyone I know, he has bridged that gap from basic science to application, and itís really going to pay off for patients. I’m convinced of that.”
Wright began working with the research team about five years ago, after he (continue reading)…
Repairing a stroke-damaged brain
So far, stroke is catastrophic. It torches a part of the brain, kills the tissue, and leaves nothing but a gap, an empty hole. The hole does not heal; the tissue does not regenerate. If we’re lucky, brain cells may wire around the hole—neurons connecting new pathways. Sometimes, patients can regain some function. Too often, they can’t.
As of today, there is only one FDA-approved clinical treatment, an enzyme known as tissue plasminogen activator (TPA). It’s useful for only one type of stroke, and very few patients qualify. For the vast majority of stroke victims, a hole in the brain is for keeps. And so far, all attempts to fill that hole have failed.
Natasha Topoluk would like to change that, and she’s off to a good start (continue reading)…

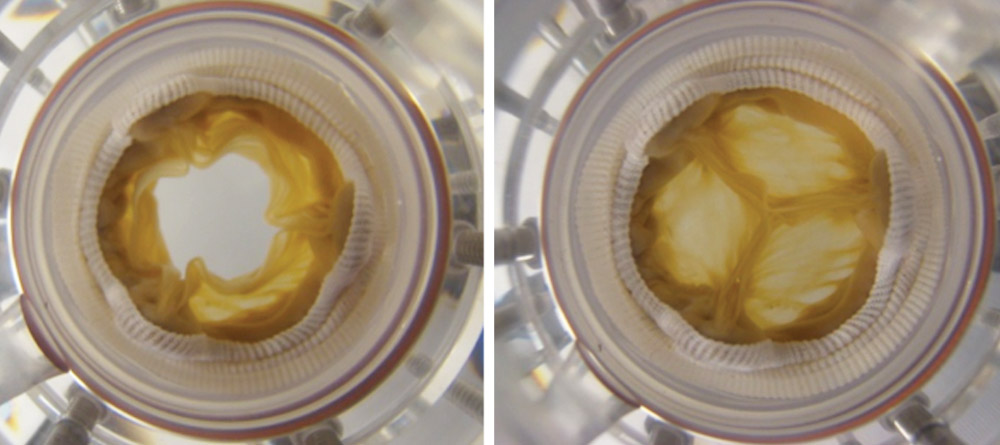
James Chow (left) and Lee Sierad (right) work on the vascular bioreactor for testing artery scaffolds, using the Patewood facility. Photo by Craig Mahaffey.
Putting new parts to the test
Lee Sierad likes to cook, so if it takes a while to launch the business he’s planning, he could always find a job in a restaurant somewhere. In high school, he worked his way up from dishwasher to line cook, so he knows his way around the kitchen. But the business he’d really like to start would design and build bioreactors, devices that could simulate conditions in the body and help bioengineers regenerate and test replacement parts. In a way, a bioreactor is a kind of test kitchen for tissues designed to help people heal.
Sierad, who is in his final year of work on a Ph.D. in bioengineering, built his first bioreactor as part of his master’s project, working with Dan Simionescu in the Biocompatibility and Tissue Regeneration Laboratories.
“It’s a system to pump fluid through a heart valve, the way it works in the body,” Sierad says. With its chambers, pumps, and valves, the bioreactor can simulate blood flow, and the pressures and rhythms of a beating heart.
But Sierad isn’t just testing new heart valves; he makes them (continue reading)…
Defending implants from diabetes
It is difficult enough to engineer a matrix populated with stem cells, and use it to replace a body part. It is difficult enough to design that matrix to fade slowly away as the patient’s own tissues and cells take over and make the part their own. But if the patient has diabetes, the degree of difficulty goes way, way up (continue reading)…
About that fear of rejection…
The Simionescus are not the first to show that the human body can readily accept an implant prepared with a matrix, and Dan says that millions of people are living proof. Two examples of common treatments: injections of bovine collagen in cosmetic surgery and implanted pig matrix for skin regeneration.
It was late, and snow flurries danced in the empty streets of Bucharest. Dan and Agneta had finally finished their work for the day—a liver-enzyme assay that had kept them in the lab until midnight. They were two Romanian undergraduates, studying biochemistry. They were falling in love.
More than three decades later, they remember that night when the world was alive with so many swirling possibilities. The world is still alive that way for them. As it is for their students. As it is for the surgeons who volunteer time to be part of their research. As it is for those who hear the story of their work.
This story is a romance. It begins with two students who fall in love and learn, together, how to mend a broken heart.
To get started, let’s go back to Romania, during the early 1980s. The newlyweds were working side by side in a lab in Targu Mures, in the heart of Transylvania. On the bench was a heart valve constructed from bovine pericardium, the sack-like tissue that covers the heart of a cow. Their boss, Radu Deac, a cardiovascular surgeon, had recruited Dan and Aggie Simionescu, offered them jobs and a lab and a house, because he believed they could help him save lives. Many of his patients needed heart valves, and Deac did not have enough valves to give them.
“He would send them home, and they would die,” Dan recalls.
In those days, he says, heart-valve replacements were new on the market, and very expensive, and the Romanian government wasn’t buying enough of them to meet the need. Deac had seen, in his travels, a new type of heart valve, designed by another Romanian, Marian Ionescu, that seemed to have promise but with room for improvement. “I know how to make these valves out of cow tissues,” Deac told Dan and Aggie, “and you can help.”
So the Simionescus, educated as biochemists, began to learn, on the job, the new skills of biomedical engineering. The work was so consuming that they could almost forget, during the long days and nights in the lab, the heartbreaking hardships of Romania in the 1980s.
Deac, stringent and exacting, continuously altered the design as the team tested and tailored each valve. “We had made for him, in the machine shop, a little device to test the valves,” Dan recalls. “We would wait for him in the evenings, and after ten hours of surgery he would come into the lab, and he would mount the valve we had made during the day, and he would test it individually, by hand, to see how it functioned. After stringent quality control, maybe one in ten valves received the stamp of approval and were prepared for implantation.”
Aggie still remembers very clearly her first glimpse of Deac’s patients. “I went into the hospital to meet with him, and I saw some of the patients in their pajamas. I felt a bit unwell, because I was very young and I had never been in that part of the hospital before. I realized for the first time who we were working for, and that this was a huge responsibility.”
Each day, the couple worked as though the patients watched them, waiting and hoping. Gradually, the tweaking and testing paid off. The heart valves were working; patients were going home to live their lives.
“It took us about five years to prepare those valves,” Dan says, “and we made about a thousand of them. So our careers started by saving a thousand patients.”
Learning from the failures
The Simionescus spent another five years helping Deac develop a method for repairing valves with bits of tissue snipped from the patient’s own pericardium, the sack around the heart. Meanwhile, a few of the patients with artificial valves implanted before 1980—twenty or thirty of the one thousand, Dan says—returned to the hospital because their replacement valves were failing. The team tried to learn from these failures and improve the new valves, a line of research that became the basis of Dan’s Ph.D. project.
“By finding out how they failed, we realized that there were ways we could make them better,” he says. “The heart valves failed because they started to calcify. You could find real stones that were built on them.”
Before they calcified, the failing valves would begin to deteriorate and thin out. Aggie, conducting research for her Ph.D., discovered that certain kinds of enzymes were degrading the tissue.
The main trouble, the couple realized, was that the heart valves they’d been making relied on tissues with dead and dying cells—the best available option at the time. In those days, the team treated the tissues with a chemical that would prevent rejection by the human body. The chemical killed the cells, but the tissues remained strong and intact, working fine for years with very slow degradation. “Once the cells were killed,” Dan says, “they became little points where calcium started to be deposited, and it grew there, like a crystal.”
So these heart valves, which had saved so many lives, were not the final answer. By the time the Simionescus found their way to Clemson—Dan in 2001 and Aggie a few years later—they were ready to try something new. They brought with them several old habits worth keeping. For one thing, they would continue to work as a team, stronger together than apart. They would continue to collaborate with surgeons— people who, like Deac, understood what patients needed. And they would work with the patient in mind.
A true romance requires more than attraction and common interests. It is a daring adventure into the future, into big, ambitious dreams. In their new country, Dan and Aggie Simionescu began to pursue a big, ambitious dream.
“Imagine,” Dan says, “that one day you could go to the hospital and have your own stem cells collected from you as a patient and that your own cells could be used to regenerate a new heart valve, new cartilage, or new tendon, and the surgeon could implant the new part in you. It would be yours, made of your own living cells. This is the future. And this is what we are doing.”
The dream is on the verge of coming true. Which brings us to the science.
The heart from Snow Creek
Romance is not always a matter of moonlight and roses. Sometimes it requires the services of a slaughterhouse, where the heart of a pig goes on ice.
Each year, the people from Snow Creek Meat Processing in Seneca, South Carolina, take a field trip to campus, to see for themselves what goes on with the products they pack up and send to the lab. Take the pig heart, for instance. Dan will make use of its valve. His students will cleanse it with detergents, wash away its cells, and remove every trace of its pigness—proteins the human body would reject. What’s left when the cleaning is finished will be an empty framework, neutral and inert—a well-ordered absence of life. Call it a matrix, a lattice, a scaffold. It is a weave of tough collagen fibers, a netting that holds life together, for any sort of animal, including us.
Nature has a frugal way of reusing a structure that works, handing it down from species to species over millions of years. When that happens, biologists say the structure is well conserved. The extracellular matrix and its collagen are well conserved. Humans have it, and so do the critters around us. In the matrix, at least, we are one.
And so when the matrix is empty, our stem cells can move right in, like the next round of guests at a freshly cleaned hotel, without fear of rejection. Mi casa, su casa, the matrix says. Nothing lifelike remains there. There are no antigens to provoke an attack from the body. There are no dead cells for enzymes to degrade, no dead spots for calcification. And the stem cells make themselves at home.
Stem cells. The term may still carry baggage, for some. Not very long ago, people argued the ethics of using a particular kind of stem cells, those from human embryos. As it seemed at the time, embryonic stem cells were medical science’s best hope for regenerating tissues and organs. But over the last decade or so, scientists have learned that several types of adult stem cells, which are not from embryos, are also “pluripotent.” They can morph into multiple cell types and help generate many kinds of tissues.
Conveniently, a vast number of these pluripotent stem cells are stashed behind our bulging waistbands, in the fat below the surface of our skin. “Sometimes,” Dan says, smiling, “it’s good to have a little fat.” (Even though he, by all appearances, is lacking.) One day, he says, our fat might save our lives—assuming we don’t overdo it. (More about this later.)
If stem cells are actors waiting for their turn on stage, the fat below our skin is a cushy kind of green room. On cue, our stem cells come racing to the rescue, transforming themselves for the roles they are called on to play. For their research, Dan and Aggie can buy the stem cells they need from companies that extract them from fat removed during liposuction. But in the clinic, a surgeon would harvest a bit of the patient’s own fat, and its stem cells, through a small incision.
“From a piece of fat the size of a walnut, we can get millions of stem cells,” Dan says. “If you amplify them in the lab, you can get twenty million, a hundred million enough to regenerate a small piece of tissue.”
Dan and Aggie say that their collaborators, Jeff Gimble and Bruce Bunnel of Tulane University, have provided invaluable expertise on adult stem cells. “Every day we learn new things about the adult stem cells we find in our bodies,” Dan says.
The magic in the matrix
But tissue generation is not as simple as dosing an injury with stem cells. If a wound is massive, stem cells cannot find the remnants of structure they need to begin the repair. They float around and die. If a disease is too virulent, it overwhelms the stem cells, and they cannot thrive. The matrix, Dan says, gives stem cells a place to hole up and get ready to grow new tissue.
Which brings us to a bona fide breakthrough, a discovery that has attracted not only the attention of scientists and engineers but the passionate, personal investment of students and busy surgeons and colleagues. The Simionescus have shown that human stem cells, extracted from the fat beneath our skin, can multiply and populate a matrix, transform themselves, and begin to grow replacement parts biologically the same as our original equipment.
Somehow—and the exact how of this so far remains a mystery—the stem cells read the matrix and learn what to be. Perhaps they detect some kind of chemical signal, or perhaps they are reading the structure itself, but they get the message. Whatever destiny the matrix ordains, the cells make a lifelong commitment. They are transformed. They set off a chain of events that lead to new, living tissue. The heart valve they form doesn’t just look like a heart valve—it is a human heart valve.
“If you looked at one of these valves in a patient,” Dan says, “the only difference you’d probably see is the sutures the surgeon would use to implant it.”
In concept, all of this seems simple. But each kind of tissue is different, requiring its own specialized method for inserting stem cells into the matrix. “The process is called seeding,” Dan says, “and it takes a little bit of imagination and trial and error to learn how to put the seeds where they should be. If we do that right, in cell-culture conditions, where it’s warm and humid and the nutrients are there, the stem cells change into the right type of cells. It’s like the matrix tells them, “You should become this type of cell.”
So far, each type of tissue the lab has studied responds to this method. “Every month or so we have a new example,” Dan says. One example is an intervertebral disc, the padding between two vertebrae. “We took the discs from pigs, removed all the cells, and we put in human, fat-derived stem cells, and they became intervertebral disc cells in the lab,” Dan says. “This may help surgeons treat back pain.”
Putting the parts through their paces
The lab also makes arteries, cartilage, ligaments, skin, heart valves, and new tissue for stroke-damaged brains. Students develop and test these parts using equipment they build themselves—bioreactors that simulate conditions in the body.
“We have developed a bioreactor for each type of tissue,” Dan says. He credits Lee Sierad, a Ph.D. candidate in Dan’s lab, for advancing this “extremely challenging” part of the work (see putting new parts to the test).
“So we have a heart-valve bioreactor, for example, which allows you to take a heart valve and seed it with cells, and then subject it to the kinds of things that would happen if it were implanted in a heart,” Dan says. “Before too long—in two or three weeks—the tissue matures, the cells change into what we want. We think that this is the way we can prepare a living tissue replacement, ready for implantation.”
The bioreactor can also give the growing replacement part some fitness training. “We’ve learned that some tissues need mechanical stimuli to mature and to grow, to start to regenerate,” Dan says. “We can make a better implant by taking the cell-seeded scaffold—an artery, for example—and pulsating it mechanically to make it ready for implant. We call it conditioning. It’s like any athletic conditioning.” Mechanical stimulation may also help teach the stem cells how to differentiate, to turn into a particular type of tissue, he says.
Through all these steps, surgeons from the Greenville Health System track progress, advise students, and set goals for the work. (See surgeons help guide research). There are ten of them, at the moment, and they are all volunteers who, as Deac did, see promise in this kind of research. Sometimes the surgeons come to campus to meet with the team or assist with implants in the animals used for testing. Other times, they work with the team in a lab at Patewood, officially the Clemson University Biomedical Engineering Innovation Campus, a joint venture with the Greenville Health System.
The surgeons are essential to the research, Dan says. “Biomedical research has to come from the clinic. It cannot be the other way around. We go and talk to the surgeons, and they tell us about their biggest challenges. If you ask a vascular surgeon, for example, he says, ‘Well, the obese, diabetic patient has no arteries. All of them are calcified; they’re gone. Can you give us a product, because there is nothing on the market?’ So we go to the lab, and we get started.”
More than a dose of green tea
I said earlier that our fat could someday save our lives, assuming we don’t overdo it. When we lard ourselves with too much fat, we are asking for a world of hurt, especially from diabetes. Obesity and diabetes are the twin scourges of our era, an epidemic growing worse. Uncontrolled diabetes lays waste to the body, calcifying and destroying blood vessels and arteries, killing tissue, ending lives.
As Dan’s lab assembles and tests new tissues, Aggie concentrates on the formidable problem of how to regenerate tissues that can repair what diabetes has wrecked. It would do little good to implant a new artery in a diabetic patient, if a toxic soup of fats and sugars and cross-linked proteins quickly attacked the new tissue and turned it to stone. We hear a great deal about the dangers of oxidation, these days, and find ways to pack antioxidants into our diets. Diabetes unleashes a storm of oxidation, and a dose of green tea is not enough quell the storm.
Even so, antioxidants might have value in tissue generation, Aggie thought. She and her students, including James Chow who expects to finish his Ph.D. this spring, began to study an antioxidant known as PGG (pentagalloyl glucose), a natural polyphenol used in herbal remedies for various diseases, including diabetes (see defending implants from diabetes). The team began treating the extracellular matrix with PGG and implanting the matrix under the skin of rats with diabetes. The matrix survived. Better yet, when Chow populated the treated matrix with stem cells, the tissue developed normally. The implications are enormous: It might indeed be possible to implant replacement parts that could repair and resist the ravages of diabetes.
The success was not due to PGG treatment alone, Aggie says. “The stem cells have a very good effect, an anti-inflammatory effect. In tissue engineering, you want a little bit of inflammation, because you want stem cells to come in and start remodeling your tissue, but you don’t want this to happen too quickly.”
Ideally, regenerated tissue would not rely forever on the matrix used to build the implant. Instead, it would begin to regenerate its own matrix, replacing or extending the implanted one. (The Simionescus often use the word scaffold instead of matrix, to suggest the analogy of building a house: After the house is built, the scaffold can come down.) Aggie’s team has found that a recently discovered type of cell assists in matrix regeneration: the type II macrophage.
“For a very long time,” Aggie says, “we thought there was only one type of macrophage, but now we know there are two types.” Type I actually increases inflammation, because its role is to degrade tissue and clean away debris. But type II macrophages help with healing and regeneration, and they seem to be attracted by stem cells. “We believe that these stem cells send signals to these good macrophages and start regeneration,” Aggie says.
But in the case of diabetes, stem cells and their allies aren’t sufficient on their own. They need the safe haven of a matrix treated to withstand the onslaught of calcification. So in tests with laboratory animals, the implants that fared best were those with a PGG-treated matrix populated with stem cells.
All of this makes Aggie hopeful that patients with diabetes may eventually have the replacement parts they need. And this, in the end, is her goal. Ever since that evening when she first walked the floor of a hospital ward, she has remembered the point of it all: the patients.
The Valley of Death
No romance can run its course without facing a peril, a nemesis to fight. The Simionescus have never had it easy—in Romania, in finding their way through a new country and a new culture, or even in science, which is always a struggle with setbacks and complications. But they have collaborators who can help them over the technical hurdles—experts in stem cells or the extracellular matrix, surgeons and engineers, for instance. The peril they dread most, at this stage, is the Valley of Death.
They do not mean Death Valley, the football stadium. They mean a chasm that yawns between success in the lab and success in the clinic. As they watch their projects march forward, yielding heart valves and intervertebral discs and tendons and arteries and so much more, they know they are nearing the edge. They will come to a halt at the Valley of Death.
Here is how it works, as Dan explains it: “If you look at the timeline for a product going into a patient, it’s split in two parts. The first part is what you’ve heard about, the research and work in the lab. The second part is clinical trials, testing in patients. In between the two parts is the Valley of Death. And it’s scary. Why? That’s where you’re supposed to do the large-animal testing. The first part can be covered by federal funds. But before you can go into clinical trials, you have to test the products in large animals—pigs, dogs, or sheep, for example. The FDA requires that you do this to prove safety, efficacy, and feasibility. But federal agencies rarely provide grant money for large animals; it’s very difficult to get, and the work is very expensive. And usually companies will only fund clinical studies, when the product is ready for patients. We can do work with rats and mice, implant the tissues under the skin and detect if it’s antigenic, if there’s a reaction. We do this all the time. But then we get stuck. We need a hundred thousand dollars just to run one experiment with ten heart valves in ten sheep. Where do we get that?”
These days, Dan and Aggie are looking for ways to build a bridge across the Valley of Death, working every angle they can find. They spend much of their time writing grants, fighting odds in a time when federal funding is iffy and scant. In passing, Dan even wonders aloud whether people who love football might also like to help repair the injuries it inflicts upon tendons, ligaments, and cartilage.
The most promising prospect so far, ironically enough, has come from where the Simionescus began—Romania. “We applied for a grant, and we got a million euros ($1.5 million dollars) from the Romanian government to test our technology over there,” Dan says. “We are working with an amazing group of surgeons, veterinarians, and biologists who will help us implant heart valves in sheep.”
Last year, six of these scientists came to Clemson for several weeks and studied the technology.
If those implant studies turn out well, the team could possibly win approval for compassionate implantations—heart valves for human patients who would die without them. And if that works out, maybe, just maybe, American companies would see the potential, would invest in more large-animal studies to help bridge the Valley of Death.
Into the quest of their lives
The Simionescus want this success for the surgeons and their patients, certainly, but they also want it for their students. They want their students to see their hard work cross the valley, to reach the clinic and begin saving lives.
When they arrived at Clemson, the Simionescus were first and foremost researchers and problem-solvers, but now they are teachers as well. When Aggie describes her first experience in the classroom, it sounds very much like her first visit to the hospital ward. Once again, she confronted a daunting new responsibility, one that would change her life.
“Until I came here, I very rarely taught,” she says. “At Clemson, the bioengineering students had to take a tissue-engineering course, so Doctor LaBerge [Martine LaBerge, professor and chair of bioengineering] told me, ‘You are doing tissue engineering; do you want to teach it?’ So I said sure. But then once I began to prepare the course, I was very nervous at the beginning. It was a big responsibility.”
Tissue engineering is a complex field that incorporates multiple disciplines—chemistry, biochemistry, biology, engineering, physics, and more. Students take classes in these subjects, and Aggie helps them pull the pieces together and apply what they’ve learned. But as she teaches, she listens. Students bring energy, enthusiasm, imagination, and ideas.
“I always listen to the students,” Aggie says. “I take that part very seriously. They are so smart, and they have great ideas. I just love the students.”
In both Simionescu labs, undergraduate students from several departments work side by side with the graduate students, participate in group meetings, and appear as coauthors in publications. “This way we educate the next generation of scientists,” Dan says. “Our student alumni are now doctors, professors, nurses, lawyers, and entrepreneurs, and more.”
And so this story, which began with the first little flurries of romance, ends with another kind of love: a passion for leading young women and men, as Radu Deac did for Dan and Aggie, into the great, swirling quest of their lives.
Dan Simionescu is an associate professor of bioengineering and Agneta Simionescu is an assistant professor of bioengineering in the College of Engineering and Science. Dan Simionescu is also director of the Biocompatibility and Tissue Regeneration Laboratory at Clemson University and director of the Laboratory for Regenerative Medicine, Patewood and Clemson University Bioengineering Translational Research Center. The Simionescu group maintains a website and has founded an online journal, Challenges in Regenerative Medicine.
Graduate students in the Biocompatibility and Tissue Regeneration Laboratory include Laura McCallum, Allison Kennamer, Chris Deborde, Natasha Topoluk, Jason Schulte, George Fercana, Michael Jaeggli, James Chow, and Lee Sierad.
Funding for research described in these stories has been provided by the National Institutes of Health (NIH RO1 grant for heart valve regeneration, NIH R21 grant for blood vessel tissue engineering, NIH R21 grant for tissue engineering in diabetes, NIH FIRCA grant for vascular graft testing in animals), from the Hawkins Foundation of the Carolinas (for tendon regeneration), and from the Romanian Research Ministry (PCCE grant for testing tissue-engineered heart valves in animals).
Many of the projects mentioned in Glimpse are patented or patent-pending technologies available for licensing and are managed by the Clemson University Research Foundation (CURF). CURF is a nonprofit corporation that facilitates the transfer of Clemson University’s intellectual property to the private sector for commercial development and societal benefit. For further information on these or other Clemson technologies, please visit www.clemson.edu/curf or email contactcurf@clemson.edu.
Collaborators, past and present
Clinical and medical collaborators:
Eugene Langan (U.S.A., vascular surgery), Richard Hawkins (U.S.A., orthopedic surgery), Chris Wright (U.S.A., cardiovascular surgery), Christopher Carsten (U.S.A., vascular surgery), David Cull (U.S.A., vascular surgery), Timothy Williams (U.S.A., cardiac surgery), Alfred Nelson (U.S.A., neurosurgery), Sanjitpat Gill (U.S.A., spine surgery), Edward Bednar (U.S.A., plastic surgery), Roger Markwald (U.S.A., cell biology) Peter Zilla (South Africa, vascular surgery), Tim Pennel (South Africa, vascular surgery), Keno Mentor (South Africa, vascular surgery), Marius Harpa (Romania, cardiac surgery), Deac Radu (Romania, cardiac surgery), Michael Dandel (Germany, cardiology), Klara Branzaniuc (Romania, anatomy), Ovidiu Cotoi (Romania, cell biology), Petru Bordei (Romania, pathology), Lucian Harceaga (Romania, veterinary science), Egyed-Imre (Romania, pathology), Eugen Petcu (Australia, pathology), Georg Lutter (Germany, vascular surgery), Gino Gerosa (Italy, cardiovascular surgery), John Parrish (U.S.A., veterinary science).
Research collaborators:
Deon Bezuidenhout (South Africa), Toshia Fujisato (Japan), Tetsuji Yamaoka (Japan), Ender Finol, Jun Liao, Elisabeth (Betsy) Tedder, Ting-Hsien (Tom) Chuang, Jeremy Mercuri, Lee Sierad (Ph.D. candidate), George Fercana, James Chow (Ph.D. candidate), Jason Schulte (Ph.D. candidate), Richard Pascal, Mike Jaeggli (Ph.D. candidate), Katie Jaeggli, Natasha Topoluk (Ph.D. candidate), Grace Dion, Chris deBorde (Ph.D. candidate), Terezia Preda, Olah Peter, Nicoleta Suciu.
Undergraduate students:
Chris Albers, Cheryl Jennings, Jordan Maivelett, Caroline Addington, Lauren Marshall, Ryan Stowers, Chris Stabler, Charles Dunn, Barrett Hutto, Lauren Benner, Andrew Kiser, Cheryl Jennings, Marshall Mahoney, Josh Guo, Lee Mai, Ryan Gedney, Thomas Larrew, Henry Zhang, Chris Ferreir, Jonathan Hill, Devon Bowser, Laine Shaw, Harleigh Warner, Laura McCallum, Allison Kennamer, Lisa Larrew, Theresa Hafner, Mike Beshay, Elizabeth Fontaine, Nick Rearson, Anna Lou Carter, Ansari Ahmer, Haider Niazi, Irina Geiculescu, Jonathan Schwartz, Jose Chavez, Joshua Biggs, Kaitlin McClure, Katelyn Rye, Lauren Hemmingsen, Margarita Portilla, Rebekah Odum, Styam Patel, Thomas Cochran.