Open wide
by Neil Caudle
 Hai Yao in his lab: Knees and hips are simple, compared to the jaw, which does not have the advantage of a ball-and-socket structure. Yao’s model will predict the physical and chemical environment inside the joint—a necessary step to effective therapy, he says.
Hai Yao in his lab: Knees and hips are simple, compared to the jaw, which does not have the advantage of a ball-and-socket structure. Yao’s model will predict the physical and chemical environment inside the joint—a necessary step to effective therapy, he says.
Photo by Neil Caudle.
New research expands the science of our clicking, aching jaws.
For millions of people, the symptoms of a jaw problem—clicking or pain when they swallow or chew—are all too familiar. TMJ (temporomandibular joint) disorders affect about 10 percent of the population. Often, a problem first appears in the teenage years, goes away for a while, but reappears in middle age.
TMJ disorders can have multiple causes, but the jaw joint itself may account for as many as half of the cases, says Hai Yao, a bioengineer who studies the problem.
“This joint is unique in the human body,” Yao says. “Basically you have one piece of bone, the mandible, and you have a joint on the left and the right.”
When you open your jaw, it not only rotates in three axial directions, Yao explains, but the jaw must also move forward and backward and side-to-side. It is a true six degrees of freedom of motion. A disc-like piece of cartilage called a meniscus normally cushions and positions the mandible. But when the meniscus becomes displaced, it can pop away from the top of the mandible, making a clicking sound and straining the ligaments that hold it in place. In severe cases, inflammation from the stress and strain leads to osteoarthritis.
“Because you need this wide range of motion, you can’t use bone to confine it, so that’s why the stability is a problem,” Yao says. “Compared to the hip joint or the knee joint, it’s not very stable. In the hip joint you have the socket and the ball to lock it.”
So when the jaw joint causes problems, don’t expect to replace it as easily as you could replace a faulty hip or knee. At least not yet. While artificial jaw joints have been tested, they have not been very successful, Yao says. As a bioengineer, he is working toward ways to repair or replace defective jaw joints, but he says that success will depend on a much better understanding of the jaw’s biology than we have had in the past. The biology of the joint, he says, affects its mechanics and vice versa. Mechanical loading, as in hard chewing or grinding the teeth, can yield a biological response in several kinds of tissues. Yao and his team are working to understand this, right down to the level of the cell.
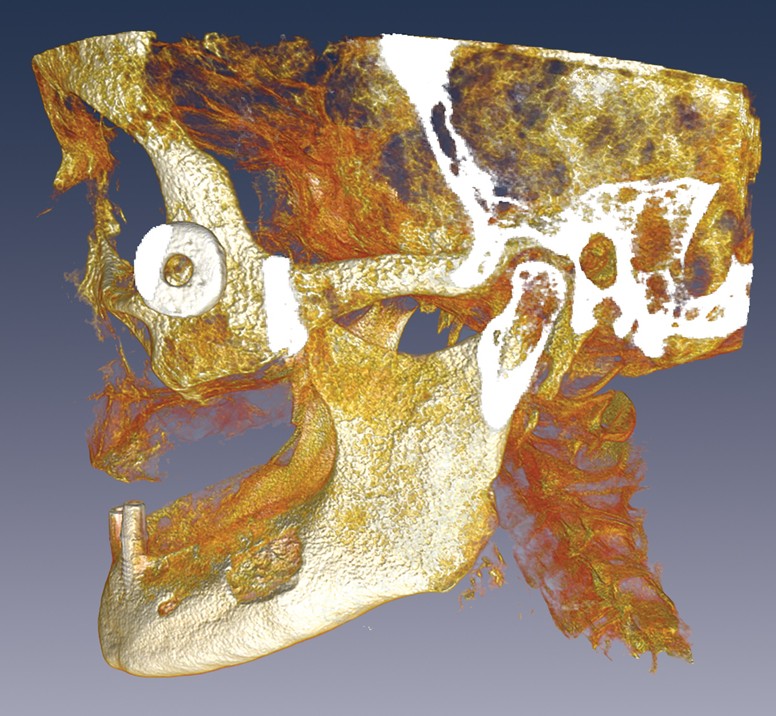
Hai Yao uses three-dimensional images of the human skull to study how the jaw works correctly—and how it fails. In this image from his lab, the TMJ is highlighted by a two-dimensional slice through the midline of the condyle of the mandible. The wheel-like form near the cheek is a marker used to align CT and MR images.
Strength and weakness in the matrix
“When you have all of this mechanical loading, you’re creating a very complex physical and chemical environment for the cell to sense,” Yao says. “The cell can sense the environment and adjust its metabolic activities.”
The cells are embedded in extracellular matrix, a kind of scaffold. It is the matrix, not the cells, providing tissue mechanical functions, Yao says. “New matrix is constantly being made by the cells, but it is also degrading, so there’s a constant remodeling,” Yao says. “This kind of modeling can maintain a balance, which is good, or it can degrade the matrix, which is bad.”
To learn how mechanical and biological factors mesh, Yao and his team study the physical and chemical environment of the TMJ and how it responds to pressure and stress. He begins with an optical system in combination of MR and CT imaging to track markers attached to key locations in the teeth and bone, and he observes what happens when a patient moves the jaw.
“If you know the motion of the teeth and the anatomy of the jaw,” Yao says, “and we can capture the true motion of the TMJ underneath the skin.”
He feeds data from this tracking system into a model that predicts various dynamic changes in the joint. “Eventually I can develop the model so that I know exactly what’s the deformation and how much stress it can generate inside,” Yao says. “Basically I will be able to predict the physical-chemical environment inside this joint.”
Surgery isn’t always the answer
With the model, Yao will investigate several unknowns about the biology of the jaw. Could deformation in the joint cause changes in the way cells embed in the extracellular matrix? Or could the changes be caused by different nutrient concentrations? Tissues in the joint have few blood vessels, he says, so cartilage, for example, relies on nutrients transported to the cells by diffusion. But various kinds of stress could suppress the flow of nutrients and degrade the tissue, weakening the joint.
The problem of blood-starved cartilage is also a frequent problem in the spine, where large intervertebral disks can degrade under stress, and Yao is studying that problem too.
Surgery, Yao says, is not always the best answer for a dysfunctional TMJ. He is developing ways to assess whether surgery makes sense in a particular case, and how it would affect the complex biology of the jaw.
Regenerating damaged tissue may yield better results than surgical intervention, Yao says. He and collaborators at Columbia University have published the results of a study that found a way to regenerate an entire joint in their animal model, the rabbit. But a successful replacement for the jaw joint may be “twenty or thirty years” in the future, Yao says.
Meanwhile, the best option for combating TMJ disorders, he says, is probably prevention. Part of his research looks at strategies for changing habits, such as clenching and grinding the teeth, that stress the joint.
Research into TMJ disorders has lagged behind other fields, Yao explains, partly because the jaw is so complex that it involves multiple kinds of expertise, each with a point of view. “You could relate it to the pain, or you could relate it to the neuromuscular factors, or you could relate it to the joint, to the tissue degeneration in the cartilage or the bone. So there’s no specific physician to take care of this problem.”
Yao draws on multiple kinds of expertise by collaborating with the Medical University of South Carolina (MUSC) in Charleston, where his lab and the Clemson-MUSC collaborative bioengineering program are based. He and his students—up to twelve of them at a time, along with several postdoctoral fellows—regularly work with physicians at MUSC. For both sides, there has been a learning curve as engineers try to acquire the languages of biology and medicine, and physicians try to understand engineering. But “Team TMJ,” as Yao puts it, is building a research program with national prominence, and the team is establishing a clinical research center with five collaborating institutions from around the country.
“This kind of research, if it eventually solves the TMJ problem, it won’t be one lab’s effort,” Yao says. “It will be a group of people.”
Hai Yao is a professor and associate chair for the collaborative bioengineering program of Clemson University and the Medical University of South Carolina.


