a sea change in shell science
by Frank Stephenson
 Andy Mount and his research team have found the “smoking gun,” the very genes that drive shell formation. “But we’re going up against seventy years of research that teaches something entirely different,” he says, “and that’s a tough thing to do.” Photo by Peter Kent.
Andy Mount and his research team have found the “smoking gun,” the very genes that drive shell formation. “But we’re going up against seventy years of research that teaches something entirely different,” he says, “and that’s a tough thing to do.” Photo by Peter Kent.
 Elizabeth Falwell, a Ph.D. candidate in Mount’s lab, examines an oyster at the edge of Winyah Bay.
Elizabeth Falwell, a Ph.D. candidate in Mount’s lab, examines an oyster at the edge of Winyah Bay.
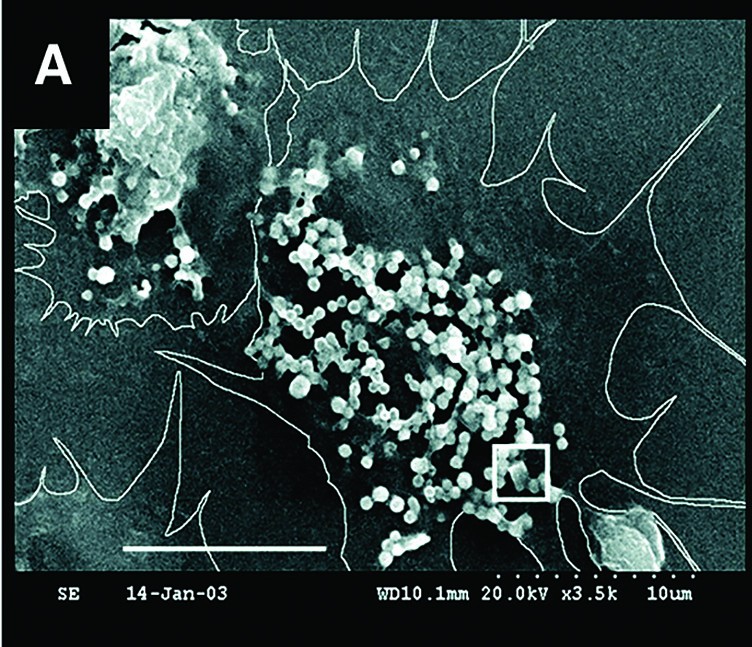 In the April 9, 2004, issue of Science, Mount published scanning electron micrographs (SEMs) revealing evidence of cellular crystal formation in oysters. In the decade since, the lab has continued to document how oyster cells make mineral “bricks” from ingredients in seawater and use them to build and repair their shells.
In the April 9, 2004, issue of Science, Mount published scanning electron micrographs (SEMs) revealing evidence of cellular crystal formation in oysters. In the decade since, the lab has continued to document how oyster cells make mineral “bricks” from ingredients in seawater and use them to build and repair their shells.
Oyster Medics
 Falwell notches the shell and watches the oyster repair the wound. Photo by Patrick Wright.
Falwell notches the shell and watches the oyster repair the wound. Photo by Patrick Wright.
Old-style oystermen have always known about it but, as is their nature, don’t make a fuss over it, or for that matter, over much of anything else an oyster does. Just part of the nature of a quiet little beast that appears to grow from nothing anyway.
Tongers—the people who use long-handled, steel-jawed rakes called tongs—know they can damage oyster reefs during periods of intense harvest. But they also know the silent beds of bivalves below them have a way of healing themselves if given enough time. As it turns out, how oysters do that is one of the great marvels in marine science.
(Continue reading at Oyster Medics.)
 Undergraduate researcher Katherine Cologne manages live oysters in the Okeanos laboratory on the Clemson campus. Image by Patrick Wright.
Undergraduate researcher Katherine Cologne manages live oysters in the Okeanos laboratory on the Clemson campus. Image by Patrick Wright.
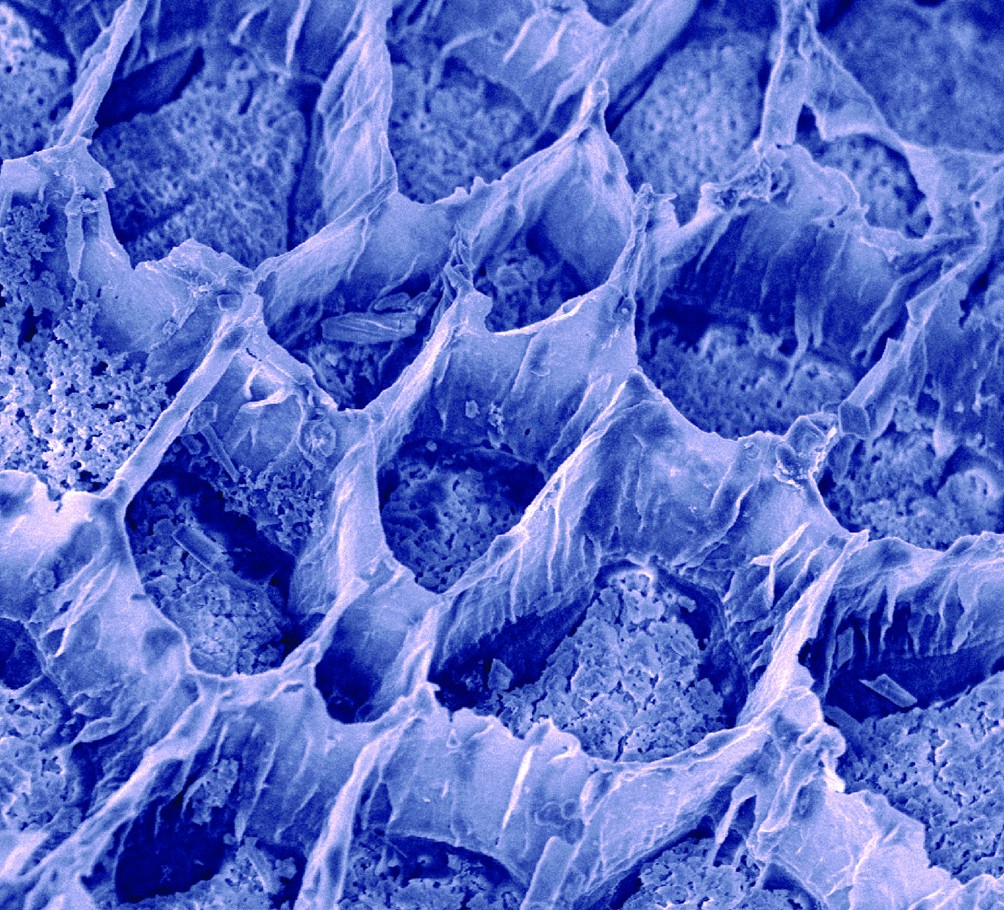 This scanning electron micrograph of the surface of an oyster shell reveals multiple strata formed through the action of the oyster’s blood cells. Image by Elizabeth Falwell.
This scanning electron micrograph of the surface of an oyster shell reveals multiple strata formed through the action of the oyster’s blood cells. Image by Elizabeth Falwell.
No harm, no foul
 Chthamalus stellatus by Michael Maggs. Image courtesy of Wikimedia Commons.
Chthamalus stellatus by Michael Maggs. Image courtesy of Wikimedia Commons.
How to stop barnacles from gaining a foothold on hulls and gear.
It’s a problem that has plagued sea-goers since the dawn of maritime history—the accumulation of barnacles, algae, and other unwanted sea growth on their boat hulls.
Even today, despite a world of technological know-how in keeping the stuff off, mariners are still dogged by the scourge of biofouling, the collective name given to the phenomenon of boats bogged down by tenacious, tag-along sea life. Fighting the problem annually costs commercial and military maritime operations billions spent on time-eating maintenance, temporary remedies—usually in the form of applying some type of toxic bottom paint—plus the extra fuel required to run fouled fleets.
(Continued in No harm, no foul.)
Artful Science
Joshua Mount, Andrew’s son, has worked in the Okeanos lab. His scanning electron micrographs, colored to distinguish the features (he calls it pseudocolor), have been displayed in galleries for their blending of science and art.
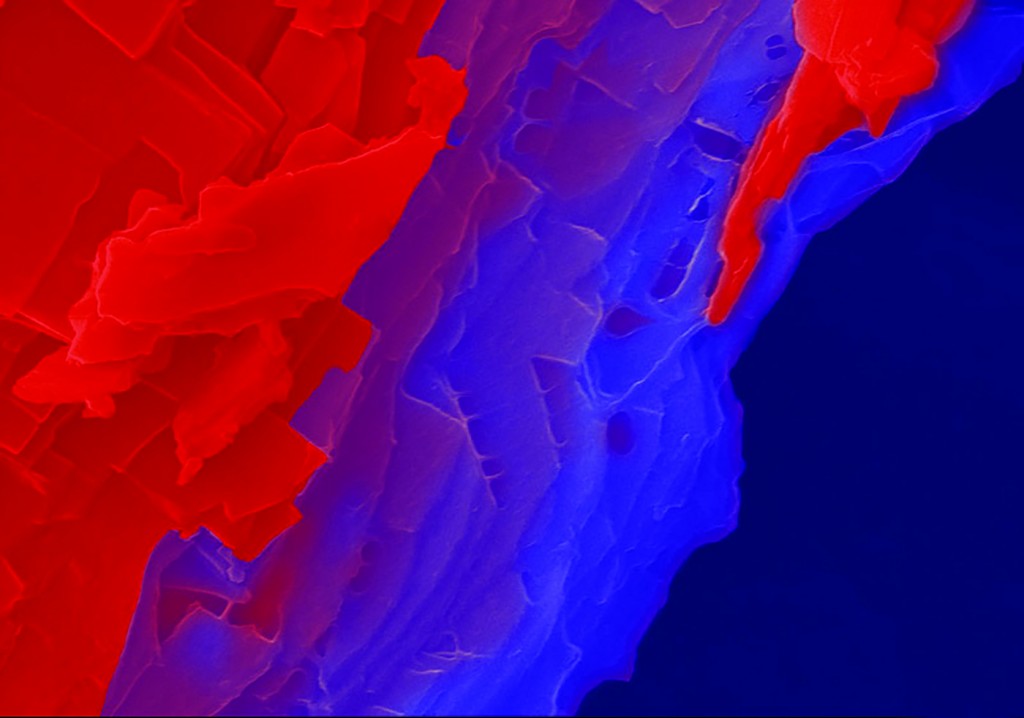 “Folia” by Joshua Mount shows the intricate mineral structures formed by organisms (biomineralization). The image shows shell mineral deposited onto a metal implant by the Eastern oyster, Crassostrea virginica.
“Folia” by Joshua Mount shows the intricate mineral structures formed by organisms (biomineralization). The image shows shell mineral deposited onto a metal implant by the Eastern oyster, Crassostrea virginica.
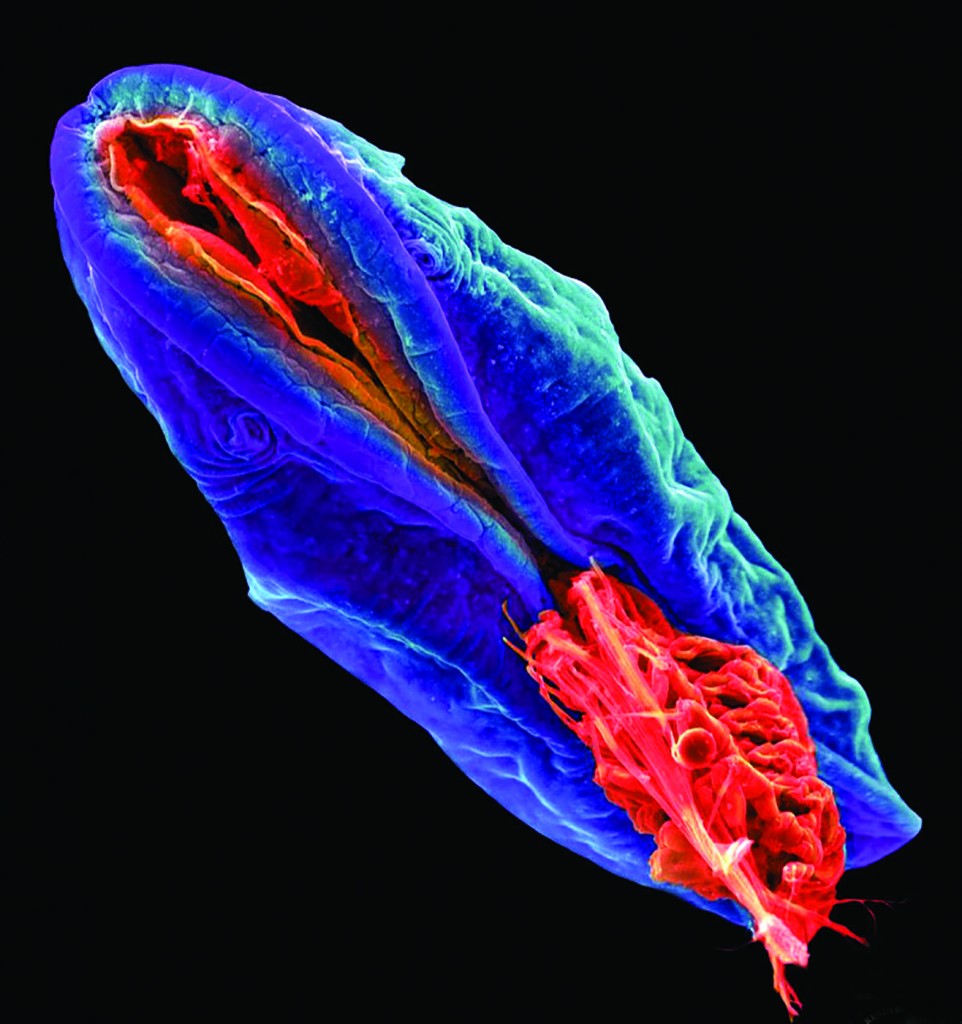 “Cypria” by Joshua Mount shows cyprid larvae of a barnacle, Amphibalanus amphitrite.
“Cypria” by Joshua Mount shows cyprid larvae of a barnacle, Amphibalanus amphitrite.
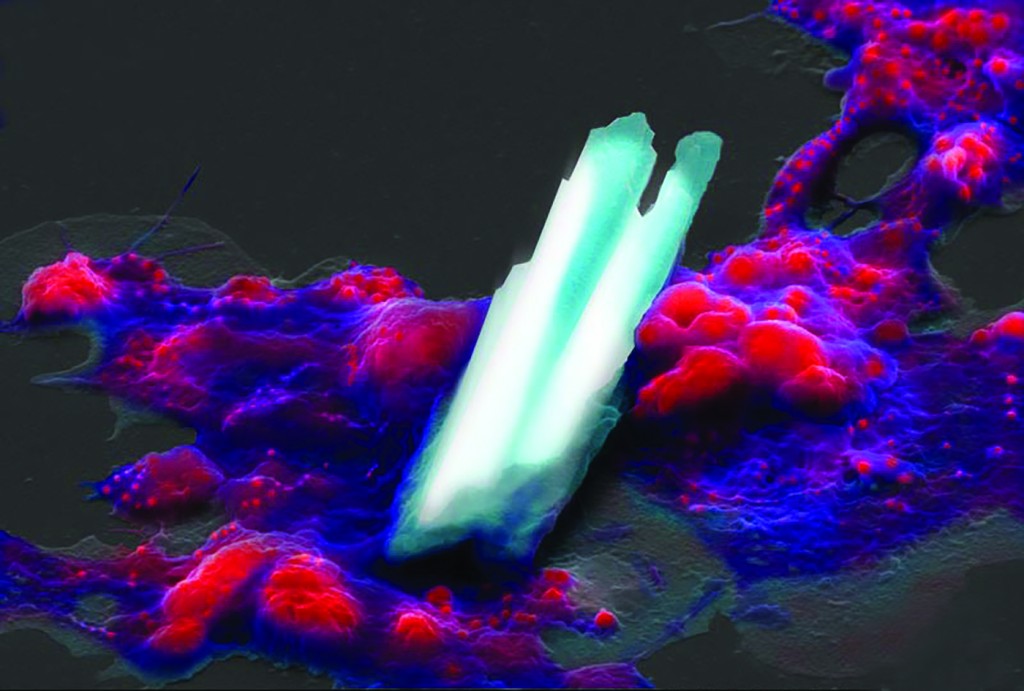 “Construction” by Joshua Mount shows an oyster blood cell, or hemocyte, manipulating calcite crystals that will later help compose a seashell.
“Construction” by Joshua Mount shows an oyster blood cell, or hemocyte, manipulating calcite crystals that will later help compose a seashell.
For more on Andrew Mount’s technology and patent portfolio and search for Mount here or contact CURF.
Debunking decades of dogma, Andy Mount and his students set the record straight on how oysters build their shells.
For starters, it’s fair to say that the man loves oysters.
Whether on a plate or a Petri dish, the briny mollusks have captivated Andrew (“Andy”) Mount since he was a kid growing up on the shores of New Jersey.
Recruited as a teen to shuck for an annual fried oyster festival at his mom’s church, Mount couldn’t have imagined he’d ever make his living as an oyster biologist. But he’s been doing just that going on three decades now, and his work is clearly paying off. He’s won a U.S. patent and has several pending applications for techniques that show commercial promise for exploiting what he’s learned about oysters’ most baffling secret, namely, how they build their shells.
Mount’s research is slowly being recognized as the dawn of a fresh, completely new understanding of shell formation in all marine animals, not just oysters. The seemingly effortless way that a myriad of sea creatures build their shells still remains much of a mystery, even though it’s common for oyster biologists to gloss over that fact when explaining how the bivalves grow. But what Mount and his team have discovered may hold the best clues yet about how oysters pull off one of the neatest feats found in nature.
Self-mending shells
Visitors to the Okeanos research lab complex on central campus aren’t there long before they catch a whiff of the ocean. In a side room, a salty tang flavors humid air hanging over a two-hundred-gallon aquarium where dozens of large oysters, imported from the Mississippi Gulf Coast, lie in a swirling bath of cool, artificial seawater. Some are busy mending their shells, deliberately cut (“notched”) to trigger their astonishing wound-healing response (see Oyster Medics).
“This is how we begin studying shell formation,” Mount says, “by notching the shells of adult oysters and studying how they heal themselves—which, I have to tell you, is quite remarkable. Our goal is to eventually study how they do it as free-swimming larvae.”
Baby oysters arise from chance meetings of eggs and sperm released into the water column by beds of adults. Larvae soon develop their own internal organs, gills, and swim gear and begin building their shells while they are still pinpoint flecks of protoplasm swimming in the open sea. These dot-sized oysters soon start hunting for a hard surface—rocks, shells, pilings, and boat hulls are choice targets—where they’ll anchor themselves for the rest of their lives. If they fail to find a good spot to settle down, they die.
A vast genetic repertoire
Beyond their famous culinary qualities, what most fascinates Mount and other Okeanos researchers about oysters is the power and agility of their genes. A specialist in cellular marine biology, Mount has managed to open a rare window into the complex world of oyster cell biology. It’s a world where a vast genetic repertoire—far richer in fact than even the human genome—builds and controls an army of proteins equipped with the muscle to do amazing things.
Just how amazing finally came to light in 2012, when an international team of scientists led by Chinese marine biotechnologists succeeded in sequencing the oyster genome, giving science its first full genetic profile of a mollusk. Published in Nature, results of the two-year study revealed the complete genetic blueprint for the Pacific oyster (Crassostrea gigas), the most common and commercially important oyster species on the planet.
Mount served as the team’s top expert on biomineralization, the catch-all term for living organisms’ immense capacity for turning carbon, calcium, oxygen, and dozens of other elements into hardened minerals for building a diverse array of bodily armament, from bones and shells to teeth and claws. Scientists have never fully understood how biomineralization works, but they do know that had the process never evolved, life on Earth would never have escaped those proverbial, primordial pools of salty water.
As the Chinese oyster-gene study began to unfold, researchers were awed by what they discovered about the oyster’s unique power to cope with life on a constantly changing Earth. Of the animal’s 28,000 genes (roughly 7,000 more than humans carry), nearly a third were found to be specifically wired to help a sessile organism—an animal destined never to move during its entire adult life—survive against the unrelenting vagaries of nature. Since the days of the dinosaurs, this oyster genome has sustained an immovable, brainless blob of protein against dramatic swings in salinity, temperature, environmental toxins, and, thanks to incessant tidal flows, frequent exposure to air and sun—lethal to the vast majority of marine organisms.
Aside from its adaptive strengths, Mount would argue that the oyster genome’s greatest triumph is its ability to wrap a spineless, soft-bodied creature in a hard, protective shell. To his delight, the Chinese-led analysis offered the first hard evidence that what he and his Clemson team had been saying for years—mostly to deaf ears—about how oysters build their shells is absolutely true.
“We made believers out of them,” Mount says, referring to his Chinese colleagues on the study. “Finally, we were able to get people to take us seriously.”
A clash of theories
In 2003, Mount’s team had made a profound discovery. The researchers uncovered solid evidence that a generally accepted theory describing how marine organisms build their shells—an idea dating to 1945—was flat out wrong.
Published in the journal Science in 2004, the Clemson paper described the discovery of a new and highly specialized type of oyster white blood cell capable of synthesizing crystals of calcium carbonate—the chief component of shells, bones, and teeth—entirely on their own and within their own cellular compartment.
The finding clashed head on with the standard model of shell formation that had dominated scientists’ thinking for nearly seventy years. The standard model holds that shell formation chiefly involves a rather strange, out-of-cellular-body phenomenon. According to the theory, when it comes time to build or repair its shell, an oyster somehow coaxes certain cells into excreting a rich, gel-like substance onto its mantle, the name for the delicate, outer layer of proteins that make up oyster “skin.” This substance contains a complex matrix of organic material that then captures all the ingredients necessary for shell construction from seawater and starts synthesizing the building blocks of calcium carbonate crystals. This “matrix-mediated” process, as it’s called, takes place entirely outside cell walls and runs autonomously, without any direct control from the oyster itself.
Mount’s discovery countered all of that. Using innovative techniques to study the insides of live cells with scanning electron microscopes, deep within what his team subsequently realized was a new type of oyster blood cell, the team found nano-sized crystals forming inside specialized cellular organs called exosomes. Okeanos researchers witnessed what no other scientists ever had—busy intracellular factories where the “bricks” of shell construction were being made. Not only that, but the research also showed in striking detail how the bricks get carried outside an oyster’s body and out to the “job site”—in other words, to wherever shell was needed, either for repair or for building an entire shell from scratch.
Normally, such a fundamental discovery would be big news in the field of cell biology. But in this case, it was anything but. Not only did the 2004 Science paper fail to make a splash in the pool of mainstream thinking, Mount’s observations—if considered at all—were dismissed as almost heretical. As a result, subsequent papers on the discovery went unpublished and Mount’s application for a patent on what he’d found triggered flags by patent officers uncomfortable with his unconventional findings.
But the oyster genome project finally won Mount and his team the vindication they had long sought. Largely on the strength of the Chinese technologists’ findings, in September 2014 the U.S. Patent Office approved a patent application covering the group’s work. The patent, prepared and submitted by the university’s Office of Technology Transfer, essentially gives Clemson a green light to license technology designed to create ultratough materials for industry using some of the same techniques that oysters have taken for granted for 200 million years.
The acid test
Biologists have reason to wonder—is there any limit to the power of proteins? Silently unfolding in their nanoworlds their myriad tasks, these muscular, molecular drivetrains put pedal to the metal at the drop of the first biochemical signal. Once fired up, they don’t stop until the job is done, whether it’s a subtle alteration in a host organism’s response to environmental change or a full-scale transformation of the organism itself.
To transform a barely visible, free-swimming oyster larva into a rock-hard bottom dweller in just a few short weeks requires protein power of the first order. It was as a grad student in Clemson’s biology department that Mount caught the fever to learn all he could about oyster proteins and, in particular, their role in building shells.
He spent six years studying proteins that he extracted by dissolving oyster shells in acid. Experiment after experiment showed the same results—the proteins seemed to be capable of cranking up the crystal-manufacturing process within individual cells. And perhaps just as astounding, these cells appeared to be crawling out of the oyster’s body and transporting crystals wherever they were needed.
“I concluded that [shell formation] wasn’t an extracellular process as the standard model had had it for so long,” Mount recalled. “It had to be coming from somewhere else in the organism.”
The Science article that appeared in 2004 was a breakthrough, but it wasn’t enough to shake the faith of most biologists in a shell-formation model that had ruled for well over half a century. Even now, after seeing his findings validated by the 2012 oyster genome project, Mount is keenly aware that the so-called “matrix-mediated model” remains the reigning paradigm. And it frustrates him to no end.
“In the Chinese study, we found the ‘smoking gun’—the very genes that drive the whole process of cellular-driven shell formation,” he says. “But we’re going up against seventy years of research that teaches something entirely different, and that’s a tough thing to do. But the fact is, once people see these data and the images we’ve captured they’re convinced.”
From the oyster genome project, one of the most powerful pieces of evidence supporting Okeanos’ work, emerged a profile done on all the animal’s proteins that were known to play a role in shell building. Researchers knew that for the standard, “matrix-mediated” model to work, oyster cells must excrete proteins to build shells. But after 259 shell-building proteins were identified, fully 84 percent were found to be proteins that never get excreted at all. If most of the shell-building proteins weren’t getting excreted, what was building the shells?
“They realized that the cells themselves were doing it,” Mount says. “We now have the genetic proof for that whole process being cellularly driven. This means that all those biologists who stuck to the old model are in trouble.”
The unobserved miracle
From one perspective, it’s hard to see what all the fuss is about. Scientists have long known about and thoroughly studied examples of entirely cell-driven mineral assembly in a veritable menagerie of marine organisms.
Plankton, the diet that supports the bulk of Earth’s marine life, is loaded with diatoms, foraminifera, and other microscopic life forms that build spectacular shells of infinite variety. One species of marine bacteria even makes crystals of magnetite, an iron-based mineral that the organism uses internally to exploit the Earth’s magnetic field (for reasons not entirely clear).
In each case, it’s a single cell doing all the work—making its own mineral “bricks” from ingredients pulled from seawater and then putting them together in bricks-and-mortar style to build their homes. The process, which mimics almost exactly what Mount has observed in oysters, is fully accepted by mainstream science.
“But when you get to a molluscan or a human system, that kind of thinking gets tossed out,” Mount says. “Conventional wisdom holds that it’s all done by a pre-formed organic matrix that gets pushed out of cells, and nature takes care of the rest by some kind of miracle. And that sort of miracle is just not observed by chemistry that I am aware of.”
A big part of the disconnect problem, Mount says, stems from the fact that the biomineralization theory sprang almost entirely from the field of geology. Working in the 1940s, geologists studying certain rocks known to have biological origins, such as stromatolites—a marine fossil found in oceans around the world—first came up with the theory. Since then, most of the refinements in the idea also have come chiefly from geologists.
Somewhat incredibly, even though biomineralization is clearly a function of living things, Mount says it is still not being studied very much from a biological standpoint. The chief biological investigators involved tend to be oceanographers and marine ecologists, whereas the core of the phenomenon lies specifically within the purview of cell biology, Mount argues.
“What’s missing in marine biology right now is that you’ve got people doing the pieces—doing ecology, biochemistry, physiology—but none of this makes sense by itself,” he says. “The only way you can begin to understand how a system works is by looking at the problem from a cellular perspective. Once you understand what’s going on in a single cell, all the other pieces fall into place. So, what’s missing in modern marine biology is that cellular approach.”
A paradigm shift
Despite being a latecomer to the debate, Mount is convinced that his team is leading a genuine paradigm shift in the science behind biomineralization. If so, he says that could have far-reaching consequences well beyond the realm of oyster biology.
“We’re revealing a new cellular paradigm of how not just oysters but how all mollusks make their shells. We see this as a fundamental advancement in the understanding of not just oysters but, frankly, of life in general.”
His point is that not only do marine organisms use cell-driven biomineralization to build shells, bones, and teeth, but that all land animals—including humans—may also be doing the same thing. Although a crystal-building cell of the type he’s found in oysters hasn’t yet turned up in the human anatomy, Mount believes it’s entirely possible that such a cell exists. It’s fun to speculate about what such a discovery could mean, he says. “If it’s true that there are human cells that make crystals like this for making bones, teeth, and so forth, that could open up the prospects of bone-healing techniques that could heal bones in days instead of weeks.”
Speculation about its implications for medical technology aside, herein lies much of the impetus behind his latest patent. While yet to draw a lot of attention, the new patent is the first to describe biomineralization techniques that in theory can be taken almost directly from the lab to the marketplace. This holds the prospect of inventing whole new technologies for building stronger and more durable materials, a main reason that Mount’s work has been supported by grants from the U.S. Air Force Office of Scientific Research. A top prospect is a tough new breed of ceramic, a synthesis of which Mount’s patent specifically describes.
“Without question, there’s tremendous potential for applications here,” Mount says. “And all we’re doing is looking at marine organisms from a cellular level. It’s exciting to see.”
Andrew S. Mount is a research associate professor with joint appointments in the Department of Biological Sciences, College of Agriculture, Forestry, and Life Sciences, and in the School of Materials Science and Engineering, College of Engineering and Sciences. He founded and directs the Okeanos Research Laboratory. Frank Stephenson (blueholeproductions.com) is a writer and editor based in Tallahassee, Florida.

An oyster reef in the North Inlet of Winyah Bay, an estuary in South Carolina. Photo by Peter Kent.


