As close as someone you care about
Peter Kent
Brian Booth is looking for a way to tame a wild child. Cancer is genetics’ wild child, relentless, growing from cell to tumor, restless, reaching from brain to bone, reckless, destroying even the body it makes home.
Actually, it’s not just one wild child; it’s a bunch of them.
Booth studies breast cancer, which killed about 40,000 women in the U.S. last year. Some 20 percent of breast cancers involved a signal for cell growth called HER2—human epidermal growth factor receptor 2. A cell biologist at Clemson’s Institute for Biological Interfaces of Engineering (IBIOE), Booth wants to know how to control HER2, which plays a major role in how cells grow and divide. Being HER2 positive means a greater likelihood that some of your cells are growing and dividing as if the accelerator pedal on a car were stuck stomped to the floor, the brakes broken and no mechanic aboard. That’s the tried-and-true analogy many physicians and scientists use to describe the spread of cancer.
The breast is a prime location for a cellular car crash, and breast cancer has been with us for a long time. An Egyptian papyrus describes eight cases of breast tumors cauterized by surgeons.
“The breast is the only organ that goes through about ninety-five percent of its development after birth,” Booth says.
When a girl reaches puberty, the hormone estrogen and other chemical signals, including growth factors, initiate and spur cell growth and proliferation. It’s a time when cells are working very rapidly and the risk of DNA damage and cell mutation is higher than normal. In a newborn, breast tissue is made of a single duct to the nipple. At puberty, the single duct branches out, creating a network as cells proliferate, grow, divide, and differentiate to make the parts of the breast.
Until a woman gives birth for the first time, the breast is more vulnerable to cancer than it will be afterward. Carrying a child to term, a woman’s breasts get different hormonal signals: immature milk-making cells respond, making milk, and the change makes the cells less vulnerable.
The two most common types of breast cancers are ductal tumors, which develop in the lining of the milk ducts, and lobular tumors, which begin where the milk is produced.
Only primates have breasts, but all mammals have mammary glands, the distinctive attribute giving our mammalian family its name. Booth uses mice, which begin puberty three weeks after birth. He inserts breast cancer cells in specially bred mice, waits for the cells to grow, euthanizes the mice, and looks at the results. Most of time the cancer cells flourish, growing, overwhelming the normal cells and spreading…but not always.
“When we mix cancer cells with normal cells, depending on the ratio of cancer cells to normal ones, we will either get a tumor or get tumor cells that incorporate into the outgrowth of mammary cells in the mouse,” Booth says. “The incorporated cells will not make a tumor. They will just become part of normal growth, become part of the cellular structure; they will even make milk.”
Why do some turn normal?
So far, these redirected tumor cells are a mystery. Why do some cancer cells begin to behave normally?
This is the question that moves Booth forward in his seat, breaks him out of his just-the-facts monotone. This is the question he thinks about all the time, even when he takes the kids to Disneyworld. “We are trying to find out what is the mechanism; why do we get tumors with some and not with others?” Booth asks. “What are the signals and what do they turn on or turn off in the tumor cells or in the normal cells that keep the tumor cells in check?”
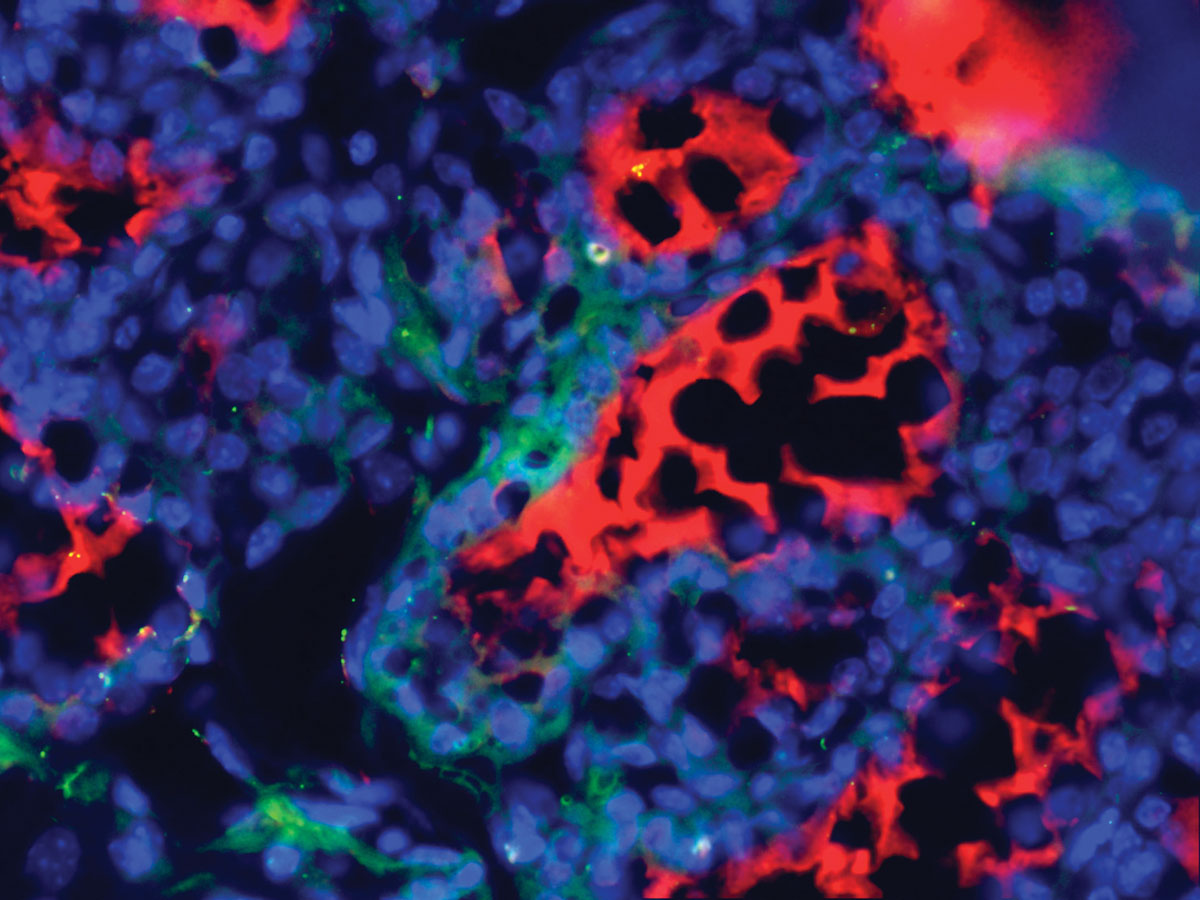 Human male cancer cells introduced into lactating mice can form a mouse mammary gland and make human milk. The blue stain indicates the cell nuclei, the red stain mouse milk, and the green stain human milk. Image provided by Brian Booth.
Human male cancer cells introduced into lactating mice can form a mouse mammary gland and make human milk. The blue stain indicates the cell nuclei, the red stain mouse milk, and the green stain human milk. Image provided by Brian Booth.Outside the IBIOE labs on the fourth floor of Rhodes Hall are stools, some of drab gray metal, others wooden with painted seats. They hold umbrellas, water bottles, and food containers—student stuff banished from the lab and offered like sacrifices. “We keep things out that could contaminate the experiments or the students,” Booth says, walking into the lab to a bench he shares with Jang Park, the postdoc who works with him. Another room has a fluorescent microscope the researchers use to look for markers, such as green fluorescent protein, that guide their search.
Like other researchers in the IBIOE, Booth is doing translational research, which means translating and applying information learned in the labs to clinics, surgery suites, and pharmaceutical makers. The tools are bioengineering and biotechnology. In the IBIOE, bioengineering can include, for example, biocompatible materials for scaffolds used in bone and tissue repairs. Biotechnology can include drug development with three-dimensional cell tissue models.
The institute and its director Karen Burg have developed a strong reputation for breast cancer research and breast reconstruction materials and techniques. “If we are successful, we see our work not as a cure but a treatment, another tool to treat cancers,” Booth says. “I have a hunch that what we will discover will not only be useful for breast cancers but other cancers, too. HER2 plays a role in cancer growth, not just in breast cancer.”
The research still has a long way to go, but Booth’s lab has isolated the redirected tumor cells and soon will begin analyzing them genetically. To do this, Booth works with Alex Feltus, a colleague at Clemson. Feltus is designing software that will rapidly sort through thousands of genetic code combinations, looking for proteins that could trigger the cellular responses Booth hopes to find. It’s a big job because cancers do not develop identically, act the same, or grow the same. There are more factors involved than oncogenes—the growth accelerators—and tumor suppressors—the brakes. Booth studies the timing and sequence of gene mutations and the messengers that carry signals inside and outside the cell. The biochemical basis for these factors is largely unknown. Booth expects that his breast cancer research will lead him to explore other cancers, because pathways involved in one cancer often are used by others.
“From the genetic and molecular profiles, we can backtrack to locate the messengers that signaled the cell, its DNA, to turn on or off the cancer cell’s growth,” Booth says. “We want to be able to do it on purpose. I believe it could be useful as either a way to reduce the need for surgery and chemotherapy or to supplement it, helping to neutralize cancer cells that may have been missed in surgery.”
HER2-positive breast cancers tend to be more aggressive than other types. There are HER2 specific drugs, such as Herceptin and Lapatinib, that kill cancer cells and lower the risk of recurrence, but 40,000 women still die from the disease and a nearly a quarter million more are diagnosed each year in the U.S. alone.
The human factor
Booth knows that cancer research can be isolating, shifting the focus from the patient to the disease. Controlled experiments, culturing cancer cells, injecting them into specialized laboratory mice, observing the tumor growths, staining slides, looking at the results through high-powered microscopes, using computers to locate and identify genes—it all but removes the human factors, which may begin with finding a lump, having it checked, and hearing a doctor say “cancer.”
“When I was doing a postdoc at the National Cancer Institute in Bethesda, there was a hospital for kids with cancer,” Booth says. “We would go to the cafeteria for lunch, and sometimes you would see the patients, the kids and their parents there. You wouldn’t eat as much; you wanted to get back to work, try a little bit harder—okay, a lot harder.”
Booth was recruited to Clemson in 2009, and there was no clinic here to remind him of the human cost of cancer. But he is a husband and a father of two girls.
He doesn’t let the question finish before answering, “Absolutely, I think about them all the time, with what I do.”
“Cancer is as close to you as someone you care about,” Booth says. His father, a retired General Motors worker, died of brain cancer in April.
As motivators, some people put up words of wisdom, others pictures of a frog or kitten hangin’ in there or a poster of a famous scientist. Booth has a newspaper clipping pinned to his office carrel wall.
The 2007 obituary tells of the life of young woman in Connecticut who died of breast cancer. Phoebe Jones Foster. Booth knew her as Phoebe Jones, two decades ago, during high school in Rochester, New York.
“She was my prom date.”
Brian Booth is a research assistant professor in the Institute for Biological Interfaces of Engineering, which spans all five Clemson colleges. Karen Burg, who directs the Institute, is the Hunter Endowed Chair and professor of bioengineering. She is also professor of electrical and computer engineering in the College of Engineering and Science. The Institute funds Booth’s research.