Self-healing plastic
Rachel Wasylyk
Scratched-up army tanks and chipped nails may one day get closure from the sun.
Learning from Mother Nature, Marek Urban has been creating plastics that are able to heal their own damage. Urban, who designs polymers and measures how they respond to chemical changes, has found ways to equip them with a built-in ability to react to a stimulus from the environment. In the process, he can fashion plastics that complete automatic functions, such as self-healing, in a controllable and predictable way.
“Mother Nature is so clever,” Urban says. “It’s inspirational from a materials point of view.” A prime inspiration is the human body, which, according to Urban, repairs approximately eighty percent of the damage it sustains. If you cut yourself, a series of reactions occur, without your having to give any conscious thought to them. Blood flows, a clot forms, and the skin heals itself. Urban has decided to mimic this process using simpler materials.
The chemistry of healing
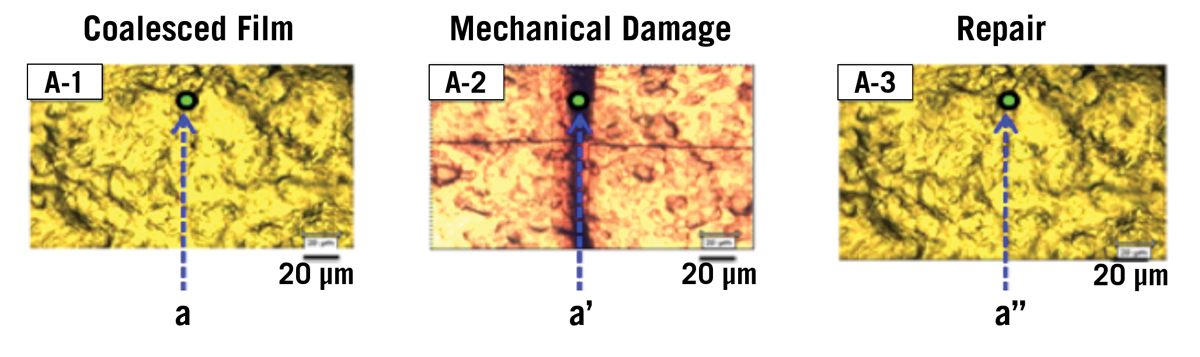
Taking inspiration from processes in living organisms, Urban has created a plastic that can mend itself when exposed to sunlight. Polyurethanes, which are polymers linked by urethane, typically make scratch-resistant materials due to their hardness and elasticity. He has modified the chemical structure to create an even more resilient substance. For the new mixture, Urban introduced chitosan, which is derived from chitin found in the exoskeletons of crabs and shrimp, to a compound called oxetane, and incorporated the new unit into the polyurethane.
He scratched the plastic and then exposed it to ultraviolet light from the sun. This energy combines free radicals, highly reactive molecules, by forming cross-links between them. As a result, the material spontaneously repairs the defect.
Another chemical approach uses copper-containing polymers that undergo charge transfers when subjected to sunlight. The energy from the light causes a change in electron placement from bonding to antibonding orbitals, thus transforming the shape of the molecule and completely restoring the plastic to its original condition.
A third form of self-healing materials actually changes colors when scratched, imitating the process of bleeding. When the substance is damaged, a chemical ring opens to form a new structure composed of hydrogen bonds. Energy from the sun is then used to break these bonds, and the polymer reverts to its original shape. The making, and subsequent breaking, of bonds temporarily creates different chemical structures, changing the color of the plastic. This was the first process that combined both mechanical and color-changing responses in the material, Urban says.
From vehicles to cosmetics
The real-world use of these self-healing plastics is diverse and widespread. “The beauty of this research is that you can always dream of more things to try,” Urban says. With a grant from the U.S. Army, the team is exploring the possibility of coating vehicles, namely army tanks, with this new material. When the transportation units are scratched or struck by rocks, they will naturally heal themselves in the sun. This could save the army money, time, and resources by eliminating a need to manually repair minor abrasions on their tanks.
The biomedical field has shown an interest in stimulus-responsive plastics for the creation of implants. The new process would both extend the life of the materials and help sustain their functions.
On a different scale, a cosmetics company plans to incorporate the new materials in a nail polish that repairs chips and blemishes when the wearer steps out in the sun. While Urban never expected this type of application for his research, he is interested in collaborating with the polish manufacturers to create a long-lasting nail wear.
Urban even hints that maybe one day we’ll be able to develop a synthetic skin-like plastic that can repair itself the same way that human skin heals, which could help with skin damaged by injury or aging. “The sky is the limit,” he says. “You can dream up and make new things through a variety of resources that are available.” Each new idea fascinates Urban, because each requires a different chemical design.
Starting from scratch
In Urban’s lab, everything is made from scratch. Rather than ordering ingredients that have already been processed, the team gets down to the most basic level, using atoms and molecules as building blocks. From here, they can build larger polymers that serve functions in specific environments. Designing plastics this way, from what Urban calls a genomic point of view, gives the researchers the flexibility to generate exactly what they intend.
But starting from scratch also requires a broad, working knowledge of the natural sciences, chemistry, and engineering. Unusual combinations of specialties have evolved in Urban’s lab to handle, for example, the chemistry behind creating polymers or the engineering of functional materials. “The key ingredient in understanding nature’s complexities and behavior is coupling between different physical and chemical processes,” Urban says. The interdisciplinary nature of research at Clemson is important, Urban says, as it creates opportunities for collaboration among departments.
“History teaches us that crossing disciplines leads to new discoveries,” he says. “One discipline can often be used to solve the other’s problems.” Rather than sending his findings off to distant locations for further testing, Urban can interact with numerous researchers on campus. As a result, his team will collaborate with others in the College of Engineering and Science and help develop the plastics they design.
On average, ten or more graduate students work in Urban’s lab, and he takes care in choosing his team. “It needs to be fun and creative for them,” he says. “It needs to fit.” With the right members, he says, no day is ever boring in the lab. “As much as you try to design experiments and think through how to do something, anything can happen and the environment changes. There is always excitement.”
He tries to teach values, along with the science. To protect the environment, his lab uses water as solvents and other sustainable practices, avoiding chemicals that cause irreversible damage.
As Urban sees it, a good way to protect Mother Nature is to emulate the way she works. “The mission of this discipline is to communicate to the public how to use plastics in a proper and respectful way,” he says. “Through this, we can save people’s lives.”
Marek W. Urban is the J.E. Sirrine Foundation Endowed Chair and a professor in the Department of Materials Science and Engineering, College of Engineering and Science. The Urban Research Group is currently funded by federal grants (the U.S. Army and the National Institutes of Health) and various private sector institutions. Rachel Wasylyk, a 2012 graduate and former editor of Decipher, a student-led research magazine at Clemson, is now a marketing coordinator and freelance writer based in Charlotte, North Carolina.