Waking from the nightmare of sleeping sickness
Jemma Everyhope-Roser
Jim Morris has found a way to kill the deadly parasite, at least in the lab. The next steps are crucial, for Africa and for science.
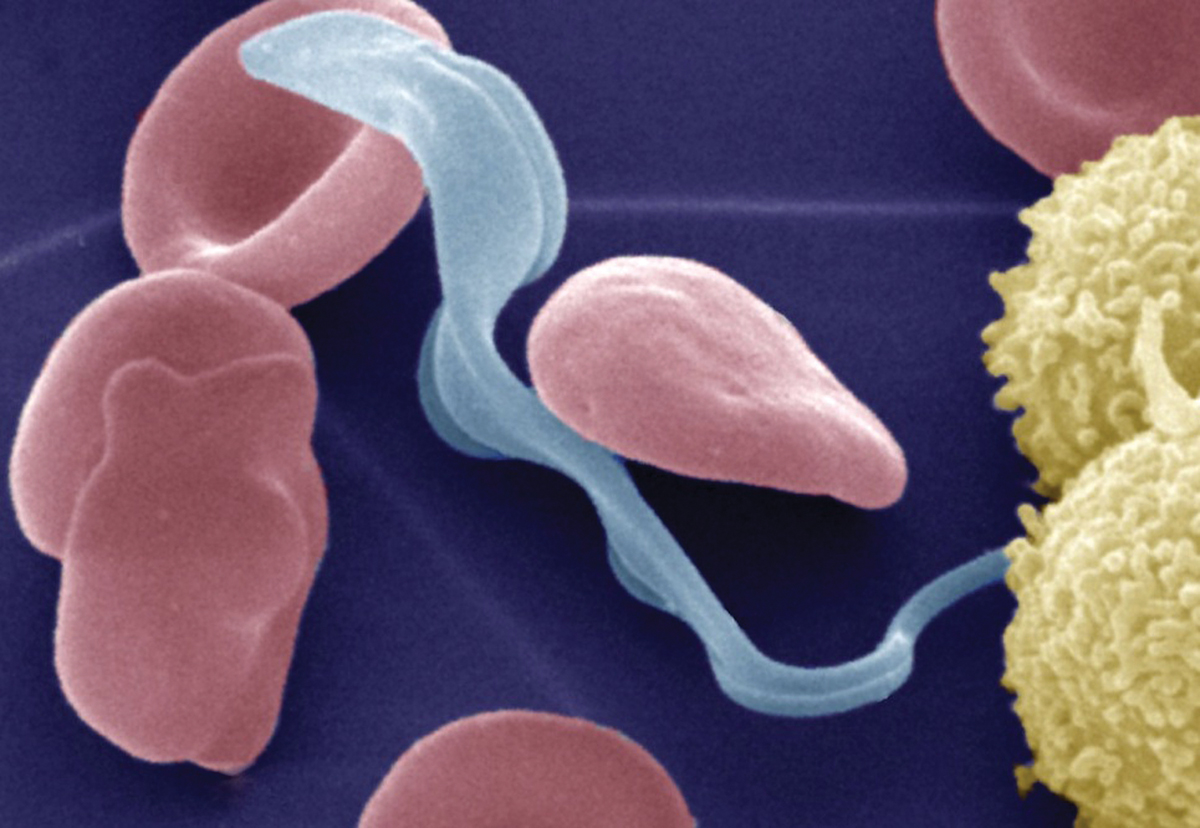
Colored electron micrograph of the bloodstream form of the Trypanosoma brucei parasite (light blue) that causes African trypanosomiasis (also called sleeping sickness) in humans in the presence of erythrocytes (red) and lymphocytes (yellow). After infection of the mammalian host by bite of the tsetse fly, the parasite lives the bloodstream before it invades the central nervous system and the brain.
You’ve heard, “Starve a fever,” but what Jim Morris is trying to starve out is African sleeping sickness. The parasite that causes African sleeping sickness is the microscopic Trypanosoma brucei. And it may have a weakness.
“There’s a very well-tuned relationship between parasite, fly, and mammal,” Morris says. When the tsetse fly bites a mammal for a delicious blood meal, it sucks up parasites especially adapted for life in the fly. The parasite then colonizes the fly’s gut. Later, some parasites will migrate through the fly’s tissue to its salivary glands. The next time the fly takes a bite out of someone, it’ll also inject parasites adapted for life in a mammal. The cycle starts over again.
“So the idea is,” Morris says, “when the parasite is migrating through the tissue of the fly, it encounters some molecule that signals the parasite is about to enter a mammal. It’s quite clear that this happens. But what the trigger is, that we don’t know.”
When Morris was a postdoc at Johns Hopkins, he used a genetic trick to identify genes involved in an important and well-understood step of the parasite’s development. And this led to them identifying genes involved in the environmental sensing. One of those genes was a metabolic enzyme, hexokinase, a protein involved in digesting food. So, the theory went, by measuring the amount of food in its environment, the parasite was able to figure out where it was.
If scientists can figure out how to disrupt this protein, they’d be able to starve the parasite, prevent it from breeding its army of clones, and trick it into believing it was safely in a fly so that our own immune systems could ambush it with impunity. That would be one effective weapon in this war.
“So the million-dollar question is how does this all work,” Morris says, “and can we find a molecule that will inhibit this enzyme?”
Cells like ours
Like many other challenges, that sounds easier than it is. The parasites are eukaryotes, higher organisms like us, and that means they operate using much of the same equipment as your standard human. Morris encounters problems similar to those seen in cancer research: how to kill cells that are similar to their human host without killing the host.
“Historically, the drugs for this have been terrible,” Morris says. “The drug that you take right now for late-stage sleeping sickness kills about ten percent of the people who take it. It’s that poisonous. It’s a tricky game, a real challenge for everyone in our field.”
Another drug, eflornithine, isn’t always even effective. Morris says, “The average patient needs several hundred grams of the stuff administered four times a day by IV. Imagine transporting that into sub-Saharan Africa. Finding a clean needle is challenge enough, but administering it four times a day sterilely…”
He fades out. It’s clear what he means. It sounds like any doctor’s nightmare.
If a person infected with the disease goes untreated, the parasites will infect the person’s brain. “It’s clear that parasites in the brain are bad for you,” Morris says. “That’s why you get late-stage disease where people are incoherent, drool, have very disrupted sleep patterns—hence the name—and your immune system mounts a very strong response. So is it your body killing your body or the parasite itself doing damage? That’s a very hard thing to distinguish.”
The good news about all of this is that the enzyme these parasites use to digest glucose is different from our own; that means it’s possible for Morris to find a compound that kills the parasite but not the person. He started his search with two hundred and twenty thousand molecules. His collaborators at the University of Virginia used robots to test the molecules’ effectiveness against the parasite’s enzyme. The best of these molecules were then passed on to other collaborators, organic chemists at the University of Kansas. The chemists “decorated” the molecules, adding or subtracting additional molecules, to see if that made the compounds more or less effective. The compounds that were effective against the parasite’s enzyme, hexokinase, they returned to Morris.
“Unfortunately, none of them killed the parasites,” Morris says. “That was a shocking finding.”
But Morris spoke with his collaborators, the organic chemists, who told him that they could tell, by just looking at the compounds, that they wouldn’t get into cells. The chemists set to work at decorating the molecules again, to make them more appealing to the parasites. Morris says, “Recently, we’ve received third-generation molecules, which now have modifications to improve cell penetration. We are now killing the parasites.”
A tough customer
But his work isn’t done yet. He has to prove that the compound works by inhibiting the parasite’s hexokinase enzyme. Morris says, a little wryly, “That’s a very difficult experiment. And we’re doing it right now, of course.”
In order to test the drug, Morris essentially has to create a parasite control group—i.e., he has to make, in his lab, parasites that are resistant to the compound. There are two ways he can do this: One way is to mutate the parasite so that it creates a resistant version of the hexokinase enzyme, and if he can do that, this would spell bad news because it would mean that it would be possible for the parasites to develop a resistance in the wild; the second way is for him to genetically modify the parasite so that it produces extra hexokinase and it could therefore survive a normal dose of the drug. In parallel to all of this, there’s also a long-term experiment running in his lab that exposes the parasites to low dosages of the drug over time; if they then develop a resistance, he’ll be able to sequence the survivors’ DNA to see how they did it.
“What I hope is that we never generate a resistance,” Morris says, “but I think that’s unlikely given how tough these guys are.”
After this, he’ll be going on to larger studies, measuring how the drug works in animals, and if they’re successful, he’ll be collaborating with a medical school. But, he says, “If we can cure animals that are affected, you have something that works in vivo. That’s a home run.”
James Morris is a professor of genetics and biochemistry in the College of Agriculture, Forestry, and Life Sciences. His research is funded by the NIH, the Clemson University Honors College, and Creative Inquiry. Jemma Everyhope-Roser is the assistant editor of Glimpse.
Colored electron micrograph of the bloodstream form of the Trypanosoma brucei parasite (light blue) that causes African trypanosomiasis (also called sleeping sickness) in humans in the presence of erythrocytes (red) and lymphocytes (yellow). After infection of the mammalian host by bite of the tsetse fly, the parasite lives the bloodstream before it invades the central nervous system and the brain.
Waking from the nightmare of sleeping sickness
Jim Morris has found a way to kill the deadly parasite, at least in the lab. The next steps are crucial, for Africa and for science.
You’ve heard, “Starve a fever,” but what Jim Morris is trying to starve out is African sleeping sickness. The parasite that causes African sleeping sickness is the microscopic Trypanosoma brucei. And it may have a weakness.
“There’s a very well-tuned relationship between parasite, fly, and mammal,” Morris says. When the tsetse fly bites a mammal for a delicious blood meal, it sucks up parasites especially adapted for life in the fly. The parasite then colonizes the fly’s gut. Later, some parasites will migrate through the fly’s tissue to its salivary glands. The next time the fly takes a bite out of someone, it’ll also inject parasites adapted for life in a mammal. The cycle starts over again.
“So the idea is,” Morris says, “when the parasite is migrating through the tissue of the fly, it encounters some molecule that signals the parasite is about to enter a mammal. It’s quite clear that this happens. But what the trigger is, that we don’t know.”
When Morris was a postdoc at Johns Hopkins, he used a genetic trick to identify genes involved in an important and well-understood step of the parasite’s development. And this led to them identifying genes involved in the environmental sensing. One of those genes was a metabolic enzyme, hexokinase, a protein involved in digesting food. So, the theory went, by measuring the amount of food in its environment, the parasite was able to figure out where it was.
If scientists can figure out how to disrupt this protein, they’d be able to starve the parasite, prevent it from breeding its army of clones, and trick it into believing it was safely in a fly so that our own immune systems could ambush it with impunity. That would be one effective weapon in this war.
“So the million-dollar question is how does this all work,” Morris says, “and can we find a molecule that will inhibit this enzyme?”
Cells like ours
Like many other challenges, that sounds easier than it is. The parasites are eukaryotes, higher organisms like us, and that means they operate using much of the same equipment as your standard human. Morris encounters problems similar to those seen in cancer research: how to kill cells that are similar to their human host without killing the host.
“Historically, the drugs for this have been really terrible,” Morris says. “The drug that you take right now for late-stage sleeping sickness kills about ten percent of the people who take it. It’s that poisonous. It’s a tricky game, a real challenge for everyone in our field.”
Another drug, eflornithine, isn’t always even effective. Morris says, “The average patient needs several hundred grams of the stuff administered four times a day by IV. Imagine transporting that into sub-Saharan Africa. Finding a clean needle is challenge enough, but administering it four times a day sterilely…”
He fades out. It’s clear what he means. It sounds like any doctor’s nightmare.
If a person infected with the disease goes untreated, the parasites will infect the person’s brain. “It’s clear that parasites in the brain are bad for you,” Morris says. “That’s why you get late-stage disease where people are incoherent, drool, have very disrupted sleep patterns—hence the name—and your immune system mounts a very strong response. So is it your body killing your body or the parasite itself doing damage? That’s a very hard thing to distinguish.”
The good news about all of this is that the enzyme these parasites use to digest glucose is different from our own; that means it’s possible for Morris to find a compound that kills the parasite but not the person. He started his search with two hundred and twenty thousand molecules. His collaborators at the University of Virginia used robots to test the molecules’ effectiveness against the parasite’s enzyme. The best of these molecules were then passed on to other collaborators, organic chemists at the University of Kansas. The chemists “decorated” the molecules, adding or subtracting additional molecules, to see if that made the compounds more or less effective. The compounds that were effective against the parasite’s enzyme, hexokinase, they returned to Morris.
“Unfortunately, none of them killed the parasites,” Morris says. “That was a shocking finding.”
But Morris spoke with his collaborators, the organic chemists, who told him that they could tell, by just looking at the compounds, that they wouldn’t get into cells. The chemists set to work at decorating the molecules again, to make them more appealing to the parasites. Morris says, “Recently, we’ve received third-generation molecules, which now have modifications to improve cell penetration. We are now killing the parasites.”
A tough customer
But his work isn’t done yet. He has to prove that the compound works by inhibiting the parasite’s hexokinase enzyme. Morris says, a little wryly, “That’s a very difficult experiment. And we’re doing it right now, of course.”
In order to test the drug, Morris essentially has to create a parasite control group—i.e., he has to make, in his lab, parasites that are resistant to the compound. There are two ways he can do this: One way is to mutate the parasite so that it creates a resistant version of the hexokinase enzyme, and if he can do that, this would spell bad news because it would mean that it would be possible for the parasites to develop a resistance in the wild; the second way is for him to genetically modify the parasite so that it produces extra hexokinase and it could therefore survive a normal dose of the drug. In parallel to all of this, there’s also a long-term experiment running in his lab that exposes the parasites to low dosages of the drug over time; if they then develop a resistance, he’ll be able to sequence the survivors’ DNA to see how they did it.
“What I hope is that we never generate a resistance,” Morris says, “but I think that’s unlikely given how tough these guys are.”
After this, he’ll be going on to larger studies, measuring how the drug works in animals, and if they’re successful, he’ll be collaborating with a medical school. But, he says, “If we can cure animals that are affected, you have something that works in vivo. That’s a home run.”
James Morris is a professor of genetics and biochemistry in the College of Agriculture, Forestry, and Life Sciences. His research is funded by the NIH, the Clemson University Honors College, and Creative Inquiry. Jemma Everyhope-Roser is the assistant editor of Glimpse.
— Jemma Everyhope-Roser
Jim Morris (right) with undergraduate researcher Katie Gray.
[photo] Craig Mahaffey
Colored electron micrograph of the bloodstream form of the Trypanosoma brucei parasite (light blue) that causes African trypanosomiasis (also called sleeping sickness) in humans in the presence of erythrocytes (red) and lymphocytes (yellow). After infection of the mammalian host by bite of the tsetse fly, the parasite lives the bloodstream before it invades the central nervous system and the brain.
Waking from the nightmare of sleeping sickness
Jim Morris has found a way to kill the deadly parasite, at least in the lab. The next steps are crucial, for Africa and for science.
You’ve heard, “Starve a fever,” but what Jim Morris is trying to starve out is African sleeping sickness. The parasite that causes African sleeping sickness is the microscopic Trypanosoma brucei. And it may have a weakness.
“There’s a very well-tuned relationship between parasite, fly, and mammal,” Morris says. When the tsetse fly bites a mammal for a delicious blood meal, it sucks up parasites especially adapted for life in the fly. The parasite then colonizes the fly’s gut. Later, some parasites will migrate through the fly’s tissue to its salivary glands. The next time the fly takes a bite out of someone, it’ll also inject parasites adapted for life in a mammal. The cycle starts over again.
“So the idea is,” Morris says, “when the parasite is migrating through the tissue of the fly, it encounters some molecule that signals the parasite is about to enter a mammal. It’s quite clear that this happens. But what the trigger is, that we don’t know.”
When Morris was a postdoc at Johns Hopkins, he used a genetic trick to identify genes involved in an important and well-understood step of the parasite’s development. And this led to them identifying genes involved in the environmental sensing. One of those genes was a metabolic enzyme, hexokinase, a protein involved in digesting food. So, the theory went, by measuring the amount of food in its environment, the parasite was able to figure out where it was.
If scientists can figure out how to disrupt this protein, they’d be able to starve the parasite, prevent it from breeding its army of clones, and trick it into believing it was safely in a fly so that our own immune systems could ambush it with impunity. That would be one effective weapon in this war.
“So the million-dollar question is how does this all work,” Morris says, “and can we find a molecule that will inhibit this enzyme?”
Cells like ours
Like many other challenges, that sounds easier than it is. The parasites are eukaryotes, higher organisms like us, and that means they operate using much of the same equipment as your standard human. Morris encounters problems similar to those seen in cancer research: how to kill cells that are similar to their human host without killing the host.
“Historically, the drugs for this have been really terrible,” Morris says. “The drug that you take right now for late-stage sleeping sickness kills about ten percent of the people who take it. It’s that poisonous. It’s a tricky game, a real challenge for everyone in our field.”
Another drug, eflornithine, isn’t always even effective. Morris says, “The average patient needs several hundred grams of the stuff administered four times a day by IV. Imagine transporting that into sub-Saharan Africa. Finding a clean needle is challenge enough, but administering it four times a day sterilely…”
He fades out. It’s clear what he means. It sounds like any doctor’s nightmare.
If a person infected with the disease goes untreated, the parasites will infect the person’s brain. “It’s clear that parasites in the brain are bad for you,” Morris says. “That’s why you get late-stage disease where people are incoherent, drool, have very disrupted sleep patterns—hence the name—and your immune system mounts a very strong response. So is it your body killing your body or the parasite itself doing damage? That’s a very hard thing to distinguish.”
The good news about all of this is that the enzyme these parasites use to digest glucose is different from our own; that means it’s possible for Morris to find a compound that kills the parasite but not the person. He started his search with two hundred and twenty thousand molecules. His collaborators at the University of Virginia used robots to test the molecules’ effectiveness against the parasite’s enzyme. The best of these molecules were then passed on to other collaborators, organic chemists at the University of Kansas. The chemists “decorated” the molecules, adding or subtracting additional molecules, to see if that made the compounds more or less effective. The compounds that were effective against the parasite’s enzyme, hexokinase, they returned to Morris.
“Unfortunately, none of them killed the parasites,” Morris says. “That was a shocking finding.”
But Morris spoke with his collaborators, the organic chemists, who told him that they could tell, by just looking at the compounds, that they wouldn’t get into cells. The chemists set to work at decorating the molecules again, to make them more appealing to the parasites. Morris says, “Recently, we’ve received third-generation molecules, which now have modifications to improve cell penetration. We are now killing the parasites.”
A tough customer
But his work isn’t done yet. He has to prove that the compound works by inhibiting the parasite’s hexokinase enzyme. Morris says, a little wryly, “That’s a very difficult experiment. And we’re doing it right now, of course.”
In order to test the drug, Morris essentially has to create a parasite control group—i.e., he has to make, in his lab, parasites that are resistant to the compound. There are two ways he can do this: One way is to mutate the parasite so that it creates a resistant version of the hexokinase enzyme, and if he can do that, this would spell bad news because it would mean that it would be possible for the parasites to develop a resistance in the wild; the second way is for him to genetically modify the parasite so that it produces extra hexokinase and it could therefore survive a normal dose of the drug. In parallel to all of this, there’s also a long-term experiment running in his lab that exposes the parasites to low dosages of the drug over time; if they then develop a resistance, he’ll be able to sequence the survivors’ DNA to see how they did it.
“What I hope is that we never generate a resistance,” Morris says, “but I think that’s unlikely given how tough these guys are.”
After this, he’ll be going on to larger studies, measuring how the drug works in animals, and if they’re successful, he’ll be collaborating with a medical school. But, he says, “If we can cure animals that are affected, you have something that works in vivo. That’s a home run.”
James Morris is a professor of genetics and biochemistry in the College of Agriculture, Forestry, and Life Sciences. His research is funded by the NIH, the Clemson University Honors College, and Creative Inquiry. Jemma Everyhope-Roser is the assistant editor of Glimpse.
— Jemma Everyhope-Roser
Jim Morris (right) with undergraduate researcher Katie Gray.
[photo] Craig Mahaffey



