How did those get in there?
Neil Caudle
Bacteria with expensive taste
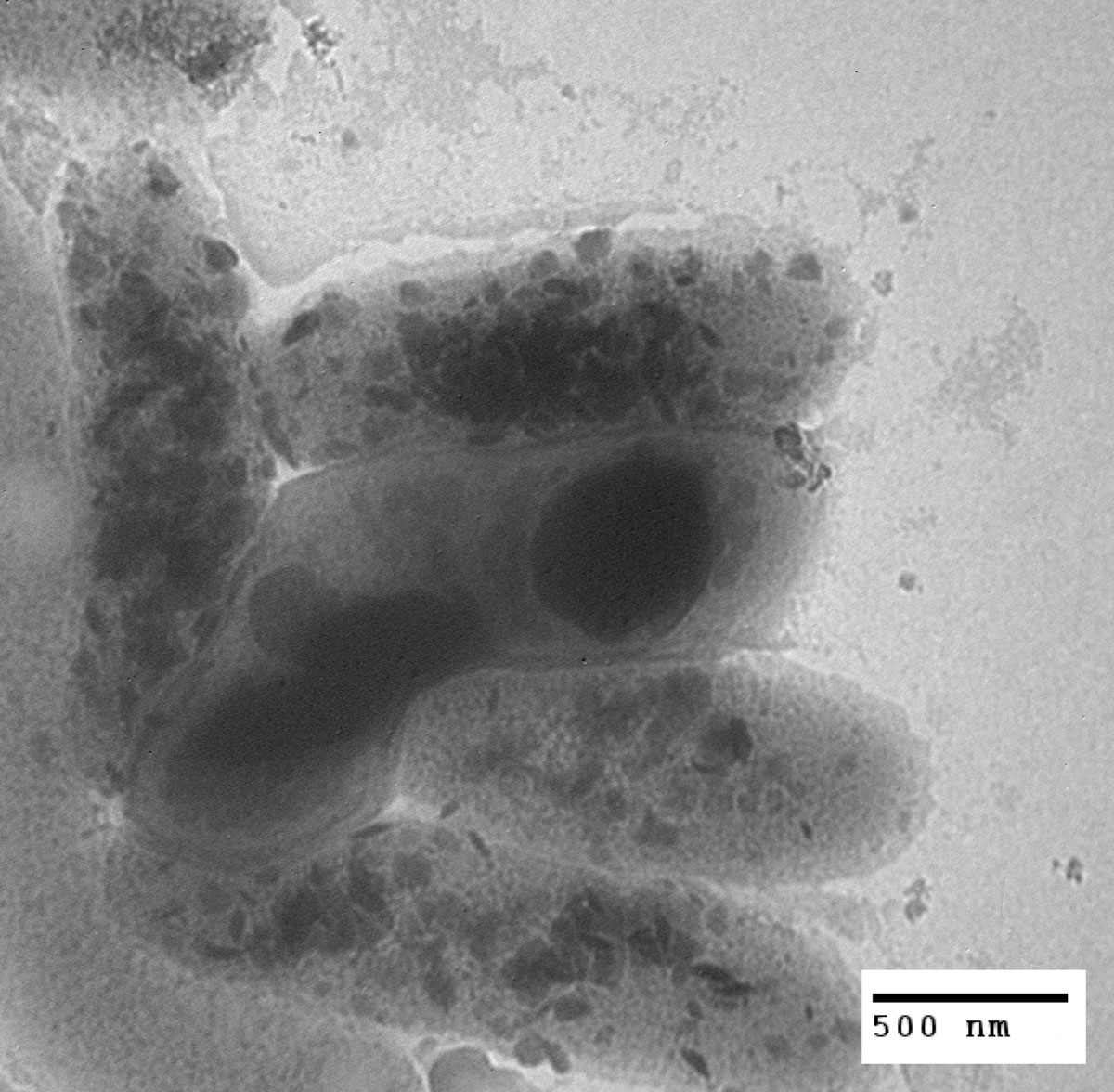
Gold nanoparticles appear as black dots inside the cells of Legionella pneumophila. How did the gold get inside? The answer could lead to new weapons against drug-resistant microbes. Electron micrograph by graduate student Amber Stojak, first published in Nanotoxicology, February 2011.
The electron micrographs seemed clear enough: Bits of gold had found their way inside living bacteria. But Tamara McNealy could hardly believe what she saw. Sure, the nanoparticles were tiny—only ten to fifty nanometers across—but they were far too large to slip through a cell membrane. If bacteria could take up particles that size, it was news to biologists. Even after two other labs published similar observations, McNealy was not entirely convinced. She asked Thompson Mefford, a whiz at crafting and using nanoparticles, to help her sort things out. If their research confirms what the micrographs appear to show, the implications are substantial, not only for science but for human health.
Runaway resistance
Medicine today faces the scary prospect of runaway resistance to antibiotics, also called antimicrobials. Resistant strains of infectious agents such as TB, staph, strep, salmonella, E. coli, and various other nasty bugs already pose a serious threat.
“One of the big problems with antimicrobials is the fact that most have to penetrate the cell wall,” McNealy says, “and bacteria develop various mechanisms to stop them.” Bacteria able to block an antibiotic survive to multiply and spread.
But if bacteria take up nanoparticles naturally, it might be possible to construct a Trojan horse, a particle that could smuggle a dose of antibiotic into the cell. One group of researchers has already shown that combining antimicrobials and nanoparticles seems to overcome certain kinds of drug resistance, but the how and why remain unknown. Maybe—and McNealy emphasizes maybe—the experimental treatment worked because the bacteria were taking up the particles, and with them the drug.
Even at a basic-science level, the discovery that bacteria take up nanoparticles is compelling. “Things don’t exist in bacteria unless there’s a natural reason for it,” McNealy says. “Many engineered nanoparticles have a natural counterpart, so there may have been a reason for bacteria to develop some kind of uptake system. Why? I don’t know.”
McNealy, a microbiologist, is the kind of basic-science researcher who loves to ask the why question. But she first began working with nanoparticles for practical reasons—to learn how various types of particles might affect bacteria.
She found that nanoparticles of gold or platinum disrupt biofilms, the slick layers of living cells that coat medical instruments and other equipment after use. Legionella pneumophila, a pernicious pathogen that causes Legionnaires’ disease, forms this kind of tough film, and scrubbing it off abrades the hardware. Conceivably, using nanoparticles to dislodge the film could help extend the life of some medical devices and allow the reuse of others that are now thrown away.

The edge of a biofilm of Legionella pneumophila, which causes Legionnaires’ disease. McNealy has found that applying gold and platinum nanoparticles dislodges the biofilm. Electron micrograph by Tara Raftery.
Risks in nature
But in nature, biofilms are not dispensable; they are necessary. Among other things, they cycle nutrients and feed amoeba and other grazers. So a second practical aspect of McNealy’s research has been the implication that releasing too many nanoparticles into the environment—from industrial sources, for instance—might damage aquatic ecosystems, and that nanoparticles taken up by bacteria conceivably could make their way up the food chain.
Having pursued these common-sense lines of applied research, McNealy now finds herself facing a fundamental question about the biology of bacteria, which is exactly the kind of basic-science question she is glad to pursue. And she happened on that question by working with scientists and materials from outside her field, pursuing new applications for nanotechnology. “It’s allowed us to discover things,” she says. “It’s allowed us to observe phenomena that we didn’t even believe were possible before.”
Tamara McNealy is an assistant professor in the Department of Biological Sciences, College of Agriculture, Forestry, and Life Sciences.
For more, please go to www.microbesadapt.com.



