a valve for saving your heart
Rachel Wasylyk
When Snow White’s hunter used a swine heart to fool the malicious queen, his scheme wasn’t very far-fetched. In its structure, a pig’s heart is similar to a human heart. But the fairytale similarities end there. While doctors have been using aortic valves from pigs for decades, approximately half of these devices fail within five to fifteen years after they’re implanted in patients. For twenty years, Naren Vyavahare has been dedicated to improving the treatments and procedures surrounding artificial heart valves.
A pressing need
Every year, more than 300,000 patients undergo replacement surgery after structural failure of their heart valves. The human heart consists of four valves that open and close with every drumming beat. The movement of the tissue flap regulates the direction of the blood, ensuring no backflow. When someone suffers from severe heart-valve disease, the structure must be replaced by an implant. With life expectancies on the rise, the number of surgeries is likely to increase, Vyavahare says.
Through his research, Vyavahare finds ways to improve heart-valve implants. One treatment he helped develop several years ago is currently being used on implants that replace defective valves. Today, Vyavahare and his team are striving to create a new technology that would increase the functional lifetime of the device, thus allowing doctors to use tissue-based valves in younger patients.
Seeing the options
There are currently two forms of artificial heart valves on the market: mechanical and bioprosthetic. Mechanical valves are constructed around a metal ring and have been shown to withstand a lifetime of physical stress. Operating in the presence of a constantly beating organ, these structures can effectively resist degeneration. But patients with mechanical heart valves are required to take daily anticoagulants, drugs that thin blood, to prevent formation of blood clots on the heart-valve implant. While the consumption of this medication every day can be an inconvenience for some, it’s not even an option for others. For women of childbearing age, the drugs interfere with contraceptive use and are also harmful to a developing fetus. In addition, patients have to undergo a monthly test to assess their blood’s clotting ability.
In the 1970s, an alternative to mechanical valves was developed. Bioprosthetic heart valves (BHVs) are tissue-based implants that are fabricated from either a pig’s aortic heart valve or cow pericardium, a thin membranous sac enclosing the heart. These devices are extremely compatible with the human body as they present little risk of blood clotting, and patients only remain on medication for a week following the implantation. But BHVs are far from perfect. Because they are composed of natural tissue, the lifetime of these devices in a human is limited. For young patients, a valve failure would require a second open-heart surgery—an experience no one wants to repeat.
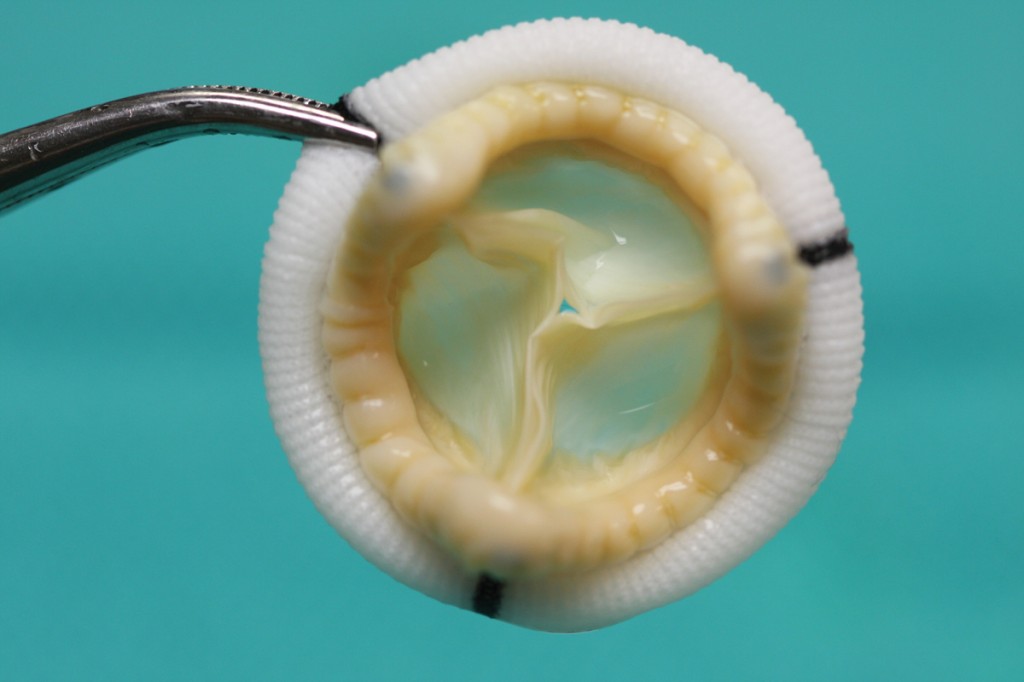
A bioprosthetic heart valve has been fabricated from a pig’s valve using the new cross-linking method developed in Vyavahare’s lab. Image credit courtesy of the Naren Vyavahare lab.
What prevents a heart from beating?
Vyavahare’s research primarily focuses on bioprosthetic heart valves and the two main issues related to their failure: degeneration and calcification.
These structures, although once alive in animals, are chemically fixed to prevent immune rejection of the tissue, and the cells are no longer living. Degeneration occurs as bending forces in the heart cause expected wear and tear on the valve. With constant beating, the device begins to degrade and cannot repair itself.
In a living cell, approximately 10,000 times less calcium can be found in the interior of the cell than outside the cell membrane because the live cell actively removes this molecule.
But in bioprosthetic valves, the dead cells aren’t able to do the same. Calcification occurs as calcium phosphates build up within the bioprosthetic tissue and cause the structure to stiffen and tear, eventually leading to valve failure.
From pigs to humans
A bioprosthetic heart valve’s extracellular matrix is comprised of three main components: collagen, elastin, and glycosaminoglycans (GAGs). Collagen fibers provide strength to the overall structure while elastin molecules lend the valve elasticity. Finally, GAGs act as a cushion between the collagen and elastin layers.
Pig-derived tissue undergoes numerous chemical treatments before it can be safely implanted in a human. Glutaraldehyde (GLUT) is the primary tissue fixative used on the valves. It has been shown to sterilize the tissue, reduce inflammation, and allow collagen cross-linking, a binding that ensures the retention and stabilization of the fibers.
The problem is, GLUT fixation makes the valve prone to calcification and degeneration. In fact, GLUT may actually increase calcium deposits in the tissue. Additionally, and most importantly, GLUT does not stabilize elastin or GAGs. Since GAGs retain impressive amounts of water, they typically provide the lubrication and cushion for a living valve. This lessens the stress induced by cardiac mechanical forces and increases the durability of the structure. Without these GAGs in place, the BHVs begin to degrade at an alarmingly faster rate, significantly shortening the overall lifetime of the device.
On the edge of discovery
Vyavahare’s team was focused on finding a chemical that would bind to GAGs, creating a cushion to protect the valve. In 2005, after several years of research, Vyavahare’s group found out that the chemical fixation of GAGs is not possible because enzymes can still degrade the GAGs in the tissue. That summer, while looking for enzyme inhibitors, Vyavahare had an idea. Neomycin, a common antibiotic, had been used in a different study where it was shown to inhibit the enzymes that degrade GAGs. What if they introduced the valve to neomycin before the GLUT? Would it have a similar outcome? Vyavahare set off to find the answer and, sure enough, the neomycin increased GAG and elastin binding and decreased the degradation of the valve during in vitro testing. If the experimental tests are successful, neomycin could have an immense impact on BHVs in the future. With added cushioning and limited calcium buildup, a valve would be able to withstand enormous amounts of stress and operate for an increased number of years.
Experimental valves were constructed and placed in sheep during the summer of 2012. The devices are scheduled to be removed soon and Vyavahare is eager to see the first round of results. But as with any research study, the goals and questions are always changing.
“A lot of optimizations in concentrations and timing are required to stabilize tissue structures without making them too stiff,” Vyavahare says, “so we’re constantly modifying our goals in order to make the best heart valves.”
Building the science
In addition to his work with bioprosthetic heart valves, Naren Vyavahare serves as the director of the Center of Bioengineering Research Excellence (COBRE). The center, funded with a highly competitive grant of $9.8 million from the National Institutes of Health (NIH), is the first and only bioengineering center of excellence in the nation.
The main focus of the center’s research is tissue regeneration. Vyavahare is a mentor for other faculty members, currently overseeing eight projects. New investigators use the center’s resources to establish their research programs and compete for their own grant funding. As they succeed, they move on and make room for additional faculty members in the COBRE center. Vyavahare says that the center is an ideal way to build the scientific community of Clemson and the state of South Carolina.
Protecting hearts
Because of the risks and complications, open-heart surgeries are a last resort. Another area of Vyavahare’s research focuses on improving BHVs that are used in procedures that access the cardiac muscle through the patient’s skin. When a person’s health prevents them from safely undergoing open-heart surgery, doctors can use a catheter to implant an animal-tissue derived valve. Although these structures have to be compressed to the diameter of a catheter, they must also possess the ability to retain their shape upon insertion. So the valve must have a combination of strong collagen and resilient elastin fibers to survive this drastic transformation.
Today, most of these devices are fashioned out of cattle pericardium. But Vyavahare’s studies determined that tissue from a pig’s vena cava, a large vein connected to the heart, would be more suitable for the job. The thin tissue consists of highly aligned fibers and an abundance of elastin, both factors lending to its ability to regain its shape after extreme stress. Additionally, the vena cava tissue showed less calcification than did the pericardium tissue. In 2011, these results were published in the journal Biomaterials and are currently being tested.
Everything takes time
According to Vyavahare, research on bioprosthetic heart valves is a very slow process. With the extensive testing required before any structures are placed in humans, it will often take ten to fifteen years before researchers know if their technology works accurately and effectively. As Vyavahare puts it, “Everything takes time.” Even after a successful field test, a new instrument may not be readily accepted by the medical community. Doctors may be comfortable with current practices and reluctant to embrace change. In addition, the research is expensive, requiring substantial, steady funding to develop the chemical treatments and new devices, with no guarantee that the technology will function correctly in the end.
But with a new, five-year, $1.2 million grant from the National Institutes of Health (NIH), Vyavahare, in collaboration with Michael Sacks from University of Texas at Austin and Joseph Gorman from the University of Pennsylvania, is working to improve degeneration and calcification issues for BHVs, as well as find new biomaterials related to these structures. Vyavahare’s goal is to create a bioprosthetic heart valve that will continue to operate thirty years after insertion in a patient.
Passing the knowledge along
Vyavahare originally planned on becoming a medical doctor. But with funding in India limited to specialized schooling, he decided to pursue a degree in chemistry instead. As a young researcher in the cardiology department at the University of Michigan, he was inspired by the work of Robert Levy, his professor and mentor. Now, over twenty years later, Vyavahare strives to have a similar influence on his students today.
Brianna Liberio, a recent Clemson graduate, says she is studying medicine in part because of Vyavahare’s example. “During my time working in Vyavahare’s lab as an undergraduate, I became more confident in a lab setting, and I am currently pursuing research opportunities as a medical student,” Liberio says. “In addition, being exposed to bioprosthetic heart-valve research sparked my interest in cardiology, and I am considering this field in my future medical career.”
This research was made possible by grant numbers NIH HL070969 and HL108330 from the National Heart, Lung, and Blood Institute at the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Naren Vyavahare is a professor and Hunter Endowed Chair of bioengineering in the College of Engineering and Science. He is also the director of the South Carolina Center of Biomaterials for Tissue Regeneration, a Center of Biomedical Research Excellence funded by the National Institutes of Health. Rachel Wasylyk, a 2012 graduate and the previous editor of Decipher, a student-led research magazine at Clemson, is now a marketing coordinator and freelance writer based in Charlotte, North Carolina.
Vyavahare’s work has led to several issued and pending patents that are available for commercialization through licensing. Contact the Clemson University Research Foundation to learn more.



